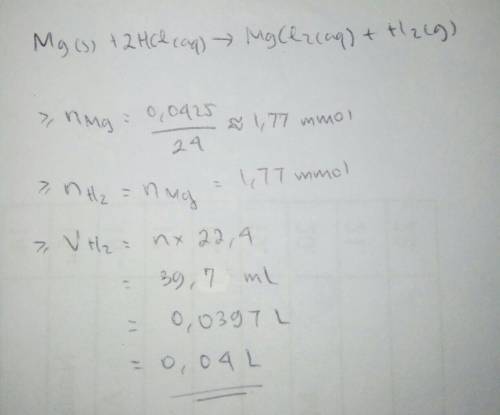Chemistry, 23.07.2019 04:00 jdkrisdaimcc11
If 0.04250g of magnesium is reacted with an excess of hydrochloric acid, calculate the theoretical volume at stp of hydrogen gas produced.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1
You know the right answer?
If 0.04250g of magnesium is reacted with an excess of hydrochloric acid, calculate the theoretical v...
Questions



Mathematics, 25.03.2021 20:20



Mathematics, 25.03.2021 20:20

Arts, 25.03.2021 20:20



Mathematics, 25.03.2021 20:20

Mathematics, 25.03.2021 20:20

Biology, 25.03.2021 20:20


History, 25.03.2021 20:20

English, 25.03.2021 20:20



Mathematics, 25.03.2021 20:20

Advanced Placement (AP), 25.03.2021 20:20

Biology, 25.03.2021 20:20