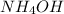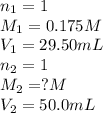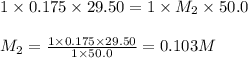Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3

Chemistry, 23.06.2019 02:00
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2

Chemistry, 23.06.2019 06:50
The student repeated the experiment using a higher concentration of acid. the same volume of acid and the same mass of magnesium ribbon were used. what volume of hydrogen gas would have been produced after 60 seconds?
Answers: 1

Chemistry, 23.06.2019 10:20
Based on the equation, how many grams of br2 are required to react completely with 29.2 grams of alcl3? alcl3 + br2 → albr3 + cl2 48.7 grams 52.6 grams 56.7 grams 61.3 grams
Answers: 3
You know the right answer?
If 29.50 ml of 0.175m nitric acid neutralizes 50.0 ml of ammonium hydroxide, what is the molarity of...
Questions


Mathematics, 17.11.2019 02:31

Mathematics, 17.11.2019 02:31


World Languages, 17.11.2019 02:31






Mathematics, 17.11.2019 02:31



Mathematics, 17.11.2019 02:31

Biology, 17.11.2019 02:31


Mathematics, 17.11.2019 02:31

Mathematics, 17.11.2019 02:31

Geography, 17.11.2019 02:31

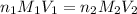
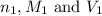 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 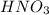
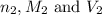 are the n-factor, molarity and volume of base which is
are the n-factor, molarity and volume of base which is