
Chemistry, 22.07.2019 14:30 b2cutie456
Identify the balanced single displacement reaction. cucl2 + fe ⟶ 2cu + feci2 pbcl4 + 2ca(oh)2 ⟶ 2cacl2 + pb(oh)4 cucl2 + fe ⟶ cu + feci2 pbcl4 + 2ca(oh)2 ⟶ 2cacl2 + 4pb(oh)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2


Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3
You know the right answer?
Identify the balanced single displacement reaction. cucl2 + fe ⟶ 2cu + feci2 pbcl4 + 2ca(oh)2 ⟶ 2cac...
Questions





Spanish, 07.07.2019 06:50




Mathematics, 07.07.2019 06:50






Chemistry, 07.07.2019 07:00





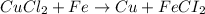
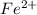 in
in 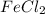 and
and 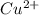 in
in 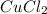 accepts electrons to form
accepts electrons to form 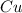 .
.
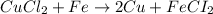 : The equation is not balanced.
: The equation is not balanced.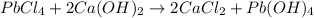 : This is a double displacement reaction and is balanced.
: This is a double displacement reaction and is balanced.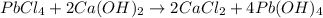 : This is a double displacement reaction and is not balanced.
: This is a double displacement reaction and is not balanced.

