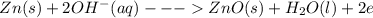Chemistry, 22.07.2019 14:30 justttlearnnn12333
Here is the discharge reaction for an alkaline battery: zn(s) + 2mno2(s) + h2o(l)\longrightarrow⟶⟶zn(oh)2(s) + mn2o3(s) which species is reduced as the battery is discharged?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2
You know the right answer?
Here is the discharge reaction for an alkaline battery: zn(s) + 2mno2(s) + h2o(l)\longrightarrow⟶⟶z...
Questions

Mathematics, 03.04.2020 02:55



Mathematics, 03.04.2020 02:55

History, 03.04.2020 02:55


History, 03.04.2020 02:55


Mathematics, 03.04.2020 02:55




Computers and Technology, 03.04.2020 02:55

Mathematics, 03.04.2020 02:55



Mathematics, 03.04.2020 02:55


Mathematics, 03.04.2020 02:56