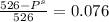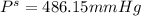Chemistry, 22.07.2019 11:30 ericasolis2614
What is the vapor pressure of a 45.0 % solution of glucose, c6h12o6, at 90.0°c, given that the vapor pressure of pure water at that temperature is 526 mm hg?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

You know the right answer?
What is the vapor pressure of a 45.0 % solution of glucose, c6h12o6, at 90.0°c, given that the vapor...
Questions

Biology, 19.05.2020 22:23

History, 19.05.2020 22:23

Social Studies, 19.05.2020 22:23



Biology, 19.05.2020 22:23



Mathematics, 19.05.2020 22:23


English, 19.05.2020 22:23

Mathematics, 19.05.2020 22:23

English, 19.05.2020 22:23




Mathematics, 19.05.2020 22:23


Computers and Technology, 19.05.2020 22:23

Mathematics, 19.05.2020 22:23

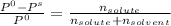 = 0.25 / (0.25 + 3.05)
= 0.25 / (0.25 + 3.05)