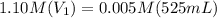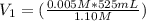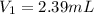Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1


Chemistry, 23.06.2019 07:30
Achemist at a pharmaceutical company is measuring equilibrium constants for reactions in which drug candidate molecules bind to a protein involved in cancer. the drug molecules bind the protein in a 1: 1 ratio to form a drug-protein complex. the protein concentration in aqueous solution at 25 ˚c is 1.74 x10-6 m . drug a is introduced into the protein solution at an initial concentration of 2.00 x10-6m. drug b is introduced into a separate, identical protein solution at an initial concentration of 2.00 x10-6m. at equilibrium, the drug a-protein solution has an a-protein complex concentration of 1.00 x10-6m, and the drug b solution has a b-protein complex concentration of 1.40 x10-6m.a. calculate the kc value for the a-protein binding reaction.b. calculate the kc value for the b-protein binding reaction.c. assuming that the drug that binds more strongly will be more effective, which drug is the better choice for further research?
Answers: 1
You know the right answer?
What volume of 1.10 m srcl2 is needed to prepare 525 ml of 5.00 mm srcl2?...
Questions

History, 05.01.2020 06:31




Mathematics, 05.01.2020 06:31

History, 05.01.2020 06:31


Mathematics, 05.01.2020 06:31

Mathematics, 05.01.2020 06:31


Mathematics, 05.01.2020 06:31

Mathematics, 05.01.2020 06:31

English, 05.01.2020 06:31

Chemistry, 05.01.2020 06:31

Mathematics, 05.01.2020 06:31

History, 05.01.2020 06:31


History, 05.01.2020 06:31


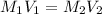
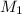 and
and 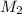 are molarities of concentrated and diluted solutions and
are molarities of concentrated and diluted solutions and 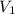 and
and 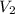 are their respective volumes.
are their respective volumes.