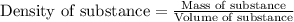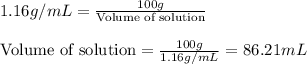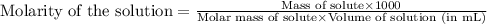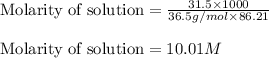Chemistry, 22.07.2019 05:00 genesisheaven1
Muriatic acid is an old name for hydrochloric acid. the commercial grade (impure) solution is still sold as muriatic acid. you use it in toilet bowl cleaners, for cleaning masonry, and for adjusting the ph of swimming pools. my local hardware store sells muriatic acid labelled as 31.5 % hcl. its density is 1.16 g/ml. assume you have 1 l of this muriatic acid (ma). determine the molarity given this information.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which statements describe polyatomic ions? check all that apply. polyatomic ions have many charges. polyatomic ions have one overall charge. polyatomic ions repel other ions to form ionic bonds. polyatomic ions attract other ions to form ionic bonds. polyatomic ions are made up of only one type of atom. polyatomic ions are made up of two or more types of atoms.
Answers: 2



Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2
You know the right answer?
Muriatic acid is an old name for hydrochloric acid. the commercial grade (impure) solution is still...
Questions


Mathematics, 06.04.2021 01:30

Computers and Technology, 06.04.2021 01:30




Mathematics, 06.04.2021 01:30

Mathematics, 06.04.2021 01:30












Computers and Technology, 06.04.2021 01:30