Chemistry, 21.07.2019 23:30 pandamaknae2003
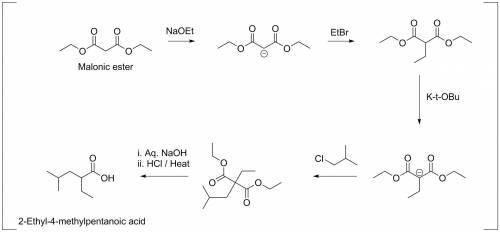
Malonic ester (diethyl malonate) is treated successively with sodium ethoxide (1 ethyl bromide, potassium tert-butoxide, isobutyl chloride, hot aqueous naoh, hcl, and heat. what is the final product?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2


Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

You know the right answer?
Malonic ester (diethyl malonate) is treated successively with sodium ethoxide (1 ethyl bromide, pot...
Questions

Mathematics, 24.03.2021 14:00



Mathematics, 24.03.2021 14:00


Arts, 24.03.2021 14:00

History, 24.03.2021 14:00







Biology, 24.03.2021 14:00

Mathematics, 24.03.2021 14:00

Biology, 24.03.2021 14:00

Mathematics, 24.03.2021 14:00

Chemistry, 24.03.2021 14:00


Mathematics, 24.03.2021 14:00