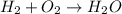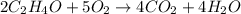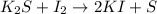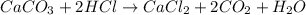Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 23.06.2019 00:30
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1

Chemistry, 23.06.2019 02:00
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1
You know the right answer?
Which of the following chemical equations is correctly balanced? a.) h2 + o2 → h2o b.) 2 c2h4o +...
Questions




English, 26.09.2019 23:30

History, 26.09.2019 23:30

History, 26.09.2019 23:30

Mathematics, 26.09.2019 23:30




Biology, 26.09.2019 23:30


History, 26.09.2019 23:30


Mathematics, 26.09.2019 23:30


Engineering, 26.09.2019 23:30



Mathematics, 26.09.2019 23:30

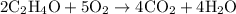 and option D.
and option D. 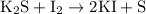 are balanced equations.
are balanced equations.