

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:50
2points what is the job of a scientist? a. to answer ethical questions. b. to write laws based on his or her knowledge. c. to ask and answer scientific questions. d. to ignore facts that do not support his or her theory.
Answers: 1

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2


You know the right answer?
Calculate the molarity of a kcl solution made by dissolving 21.2 g of kcl in a total volume of 500....
Questions




Health, 19.04.2021 20:10


History, 19.04.2021 20:10

Social Studies, 19.04.2021 20:10

Mathematics, 19.04.2021 20:10

Mathematics, 19.04.2021 20:10

Mathematics, 19.04.2021 20:10


Mathematics, 19.04.2021 20:10


French, 19.04.2021 20:10

Arts, 19.04.2021 20:10

Mathematics, 19.04.2021 20:10



English, 19.04.2021 20:10

Mathematics, 19.04.2021 20:10

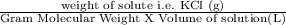
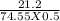 = 0.569 M
= 0.569 M

