
Chemistry, 21.07.2019 14:00 angel234wilcox
When heated, calcium carbonate decomposes to yield calcium oxide and carbon dioxide gas via the reaction caco3(s)→cao(s)+co2(g) what is the mass of calcium carbonate needed to produce 61.0 l of carbon dioxide at stp? express your answer with the appropriate units?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Motivation cannot be developed with practice; a person either possesses it or they do not.
Answers: 1

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1
You know the right answer?
When heated, calcium carbonate decomposes to yield calcium oxide and carbon dioxide gas via the reac...
Questions

Mathematics, 15.12.2020 08:00


Mathematics, 15.12.2020 08:00


Mathematics, 15.12.2020 08:00

Physics, 15.12.2020 08:00

Physics, 15.12.2020 08:00


Mathematics, 15.12.2020 08:00

Mathematics, 15.12.2020 08:00


History, 15.12.2020 08:00

Mathematics, 15.12.2020 08:00




Biology, 15.12.2020 08:00

Physics, 15.12.2020 08:00


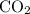 , 227.23 grams of
, 227.23 grams of 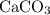 . is required.
. is required.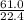
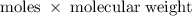
 100
100


