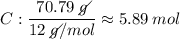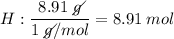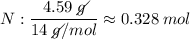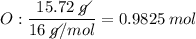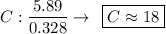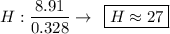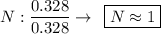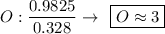Determine the empirical formula for a compound that is 70.79% carbon, 8.91% hydrogen, 4.59% nitrogen, and 15.72% oxygen. determine the empirical formula for a compound that is 70.79 carbon, 8.91 hydrogen, 4.59 nitrogen, and 15.72 oxygen. c18h27no2 c18h27no3 c17h27no3 c17h26no3

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1


Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
You know the right answer?
Determine the empirical formula for a compound that is 70.79% carbon, 8.91% hydrogen, 4.59% nitrogen...
Questions






Mathematics, 29.06.2019 23:00

History, 29.06.2019 23:00

History, 29.06.2019 23:00



Mathematics, 29.06.2019 23:00

Physics, 29.06.2019 23:00

Social Studies, 29.06.2019 23:00





Biology, 29.06.2019 23:00

Mathematics, 29.06.2019 23:00

English, 29.06.2019 23:00