
Chemistry, 21.07.2019 11:00 agarcia24101993
The voltage generated by the zinc concentration cell described by the line notation zn(s) ∣∣ zn2+(aq,0.100 m) ∥∥ zn2+(aq,? m) ∣∣ zn(s) is 14.0 mv at 25 °c. calculate the concentration of the zn2+(aq) ion at the cathode.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following true? a_volcanoes and earthquakes often near the plate boundaries. b_volcanoes occur whereve there are tall mountains. c_earthquakes cause volcanoes in the same location to erupt violently d_volcanoes and earthquakes occur only where plates are colliding with each other
Answers: 2

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

You know the right answer?
The voltage generated by the zinc concentration cell described by the line notation zn(s) ∣∣ zn2+(aq...
Questions



Chemistry, 02.12.2020 14:00


History, 02.12.2020 14:00

English, 02.12.2020 14:00

Computers and Technology, 02.12.2020 14:00


Mathematics, 02.12.2020 14:00

Biology, 02.12.2020 14:00

English, 02.12.2020 14:00

Computers and Technology, 02.12.2020 14:00


Mathematics, 02.12.2020 14:00

Health, 02.12.2020 14:00

Mathematics, 02.12.2020 14:00


Mathematics, 02.12.2020 14:00

English, 02.12.2020 14:00

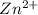 ion at cathode is 0.295 M
ion at cathode is 0.295 M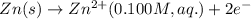
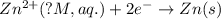
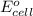 will be equal to zero.
will be equal to zero.![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Zn^{2+}]_{anode}}{[Zn^{2+}]_{cathode}}](/tpl/images/0115/3857/2e4c8.png)
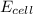 = 14.0 mV = 0.014 V (Conversion factor: 1 V = 1000 mV)
= 14.0 mV = 0.014 V (Conversion factor: 1 V = 1000 mV)![[Zn^{2+}]_{anode}](/tpl/images/0115/3857/a4cd7.png) = 0.100 M
= 0.100 M![[Zn^{2+}]_{cathode}](/tpl/images/0115/3857/54f88.png) = ? M
= ? M![0.014=0-\frac{0.0592}{2}\log \frac{0.100M}{[Zn^{2+}]_{cathode}}](/tpl/images/0115/3857/e07d0.png)
![[Zn^{2+}]_{cathode}=0.295M](/tpl/images/0115/3857/a683c.png)


