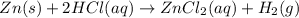Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

You know the right answer?
Given the reaction: zn(s) + 2hcl(aq) → zncl2(aq) + h2(g) the oxidation number of zn(s) increases be...
Questions


Mathematics, 12.02.2021 14:10

Mathematics, 12.02.2021 14:10

Geography, 12.02.2021 14:10

Mathematics, 12.02.2021 14:10


Mathematics, 12.02.2021 14:10


Mathematics, 12.02.2021 14:10


Mathematics, 12.02.2021 14:10


Mathematics, 12.02.2021 14:10

Mathematics, 12.02.2021 14:10


Geography, 12.02.2021 14:10

Mathematics, 12.02.2021 14:10

Mathematics, 12.02.2021 14:10