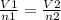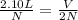Chemistry, 21.07.2019 09:00 itziabejar
If the number of moles of a gas initially contained in a 2.10 l vessel is doubled, what is the final volume of the gas in liters? (assume the pressure and temperature remain constant.) none of the above 8.40 4.20 1.05 6.30

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 22.06.2019 17:30
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1
You know the right answer?
If the number of moles of a gas initially contained in a 2.10 l vessel is doubled, what is the final...
Questions


English, 11.01.2021 17:10

Health, 11.01.2021 17:10

Physics, 11.01.2021 17:10






Spanish, 11.01.2021 17:10

English, 11.01.2021 17:10

Mathematics, 11.01.2021 17:10

Chemistry, 11.01.2021 17:10

History, 11.01.2021 17:10

Social Studies, 11.01.2021 17:10


Mathematics, 11.01.2021 17:10

History, 11.01.2021 17:10