
Chemistry, 21.07.2019 07:30 emilyanneK236
What is the emf of a cell consisting of a pb2+ / pb half-cell and a pt / h+ / h2 half-cell if [pb2+] = 0.83 m, [h+] = 0.064 m and ph2 = 1.0 atm ?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Chemistry, 23.06.2019 11:30
The dashed segment of the plotted experiment in the graph in the l
Answers: 3
You know the right answer?
What is the emf of a cell consisting of a pb2+ / pb half-cell and a pt / h+ / h2 half-cell if [pb2+]...
Questions



Mathematics, 15.10.2019 05:00


History, 15.10.2019 05:00


Social Studies, 15.10.2019 05:00


Mathematics, 15.10.2019 05:00

Biology, 15.10.2019 05:00


History, 15.10.2019 05:00

Mathematics, 15.10.2019 05:00







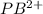 //
// 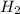 /
/ ![E^{0}cell - \frac{0.059}{n}log \frac{1}{[Pb^2^+]X[H^+]^2}](/tpl/images/0114/7789/ce948.png)
![E^{0}cell = 0.126 v\\∴Ecell = 0.126 - \frac{0.059}{2}log \frac{1}{[0.83]X[0.064]^2}](/tpl/images/0114/7789/5add0.png)
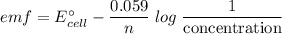
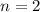
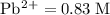
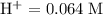
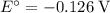
![emf=-0.126\;-\dfrac{0.059}{2}\;\times\;log\;\dfrac{1}{[0.83]\;\times\;[0.064]^2}\\ emf=0.0532\;\text V](/tpl/images/0114/7789/fcd3d.png)


