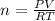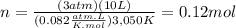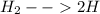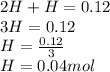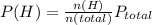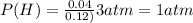Computers and Technology, 30.10.2019 06:31 jaylenmiller437
A10.0-l container is filled with 0.10 mol of h2(g) and heated to 3050 k. at that temperature, some of the h2(g) decomposes into h(g), and the total pressure is found to be 3.0 atm. find the partial pressure of the h(g) that has been formed. (keep in mind that there is a reaction taking place, so as h(g) is being formed, h2(g) is being consumed.)

Answers: 3


Another question on Computers and Technology

Computers and Technology, 22.06.2019 19:30
When creating a presentation in libre office impress, where does the editing of slides take place?
Answers: 1

Computers and Technology, 23.06.2019 02:30
What is the power dissipated by a resistor with a current of 0.02 a and a resistance of 1,000 ? a. 200 w b. 20 w c. 0.4 w d. 4 w
Answers: 1

Computers and Technology, 23.06.2019 12:00
If you're using an existing powerpoint presentation that will receive new slides based on a word outline, select the a. slide that will appear after the new slides. b. first slide in the presentation. c. slide that will appear before the new slides. d. last slide in the presentation.
Answers: 2

Computers and Technology, 23.06.2019 17:00
Companies that implement and apply an information system effectively can create
Answers: 1
You know the right answer?
A10.0-l container is filled with 0.10 mol of h2(g) and heated to 3050 k. at that temperature, some o...
Questions

















Computers and Technology, 15.10.2019 18:30