
Computers and Technology, 07.01.2020 07:31 lukeperry
A0.040 g piece of magnesium is placed in a beaker of hydrochloric acid. hydrogen gas is generated according to the following equation:
mg (s) + 2 hcl (aq) â mgcl2 (aq) + h2 (g)
the gas is collected over water at 25â°c, and the gauge pressure during the experiment reads 784 mmhg. the gas displaces a volume of 100 ml. the vapor pressure of water at 25â°c is approximately 24.0 mmhg. based on this data, how many moles of hydrogen are produced in this reaction?
a. 4.04 ã 10-5 moles hydrogen
b. 4.09 ã 10-3 moles hydrogen
c. 3.07 ã 10-2 moles hydrogen
d. 3.11 moles hydrogen

Answers: 1


Another question on Computers and Technology

Computers and Technology, 22.06.2019 06:00
What are the most likely causes of conflict at the meeting? check all that apply.
Answers: 1

Computers and Technology, 22.06.2019 17:30
The forerunner to cell phones, pdas, and smartphones was
Answers: 1

Computers and Technology, 23.06.2019 12:00
Which of these is an example of an integrated presentation? a. a table created in powerpoint b. an image pasted into powerpoint c. a caption created in powerpoint d. an excel chart pasted into powerpoint
Answers: 1

Computers and Technology, 23.06.2019 20:30
What is the biggest difference between section breaks and regular page breaks
Answers: 1
You know the right answer?
A0.040 g piece of magnesium is placed in a beaker of hydrochloric acid. hydrogen gas is generated ac...
Questions



Mathematics, 26.04.2021 21:40

Mathematics, 26.04.2021 21:40

Mathematics, 26.04.2021 21:40


Business, 26.04.2021 21:40

Mathematics, 26.04.2021 21:40

History, 26.04.2021 21:40



English, 26.04.2021 21:40

Biology, 26.04.2021 21:40


History, 26.04.2021 21:40

History, 26.04.2021 21:40


Mathematics, 26.04.2021 21:40


Health, 26.04.2021 21:40

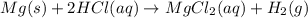
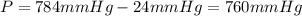
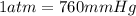
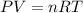 (ideal gas equation )
(ideal gas equation )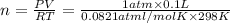
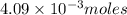
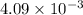 moles of hydrogen are produced in this reaction.
moles of hydrogen are produced in this reaction.

