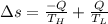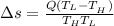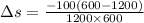Engineering, 13.07.2019 04:30 ghernadez
Heat in the amount of 100 kj is transferred directly from a hot reservoir at 1200 k to a cold reservoir at 600 k. calculate the entropy change of the two reservoirs and determine if the increase of entropy principle is satisfied.

Answers: 1


Another question on Engineering

Engineering, 03.07.2019 14:10
The y form of iron is known as: a) ferrite b) cementite c) perlite d) austenite
Answers: 3

Engineering, 04.07.2019 12:10
On a average work day more than work place firs are reorted
Answers: 1

Engineering, 04.07.2019 18:10
Which of the following components of a pid controlled accumulates the error over time and responds to system error after the error has been accumulated? a)- proportional b)- derivative c)- integral d)- on/off.
Answers: 2

Engineering, 04.07.2019 18:20
Along 8-cm diameter steam pipe whose external surface temperature is 900c connects two buildings. the pipe is exposed to ambient air at 70c with a wind speed of 50 km/hr blowing across the pipe. determine the heat loss from the pipe per unit length. (b) air at 500c enters a section of a rectangular duct (15 cm x 20 cm) at an average velocity of 7 m/s. if the walls of the duct are maintained at 100c. a) the length of the tube for an exit temperature of the air to be 40 0c. b)the rate of heat transfer from the air. c) the fan power needed to overcome the pressure drop in this section of the duct.
Answers: 1
You know the right answer?
Heat in the amount of 100 kj is transferred directly from a hot reservoir at 1200 k to a cold reserv...
Questions


Chemistry, 20.05.2021 23:40

Computers and Technology, 20.05.2021 23:40

Mathematics, 20.05.2021 23:40

Mathematics, 20.05.2021 23:40

Computers and Technology, 20.05.2021 23:40

Mathematics, 20.05.2021 23:40


Mathematics, 20.05.2021 23:40

Physics, 20.05.2021 23:40


Mathematics, 20.05.2021 23:40

History, 20.05.2021 23:40

Mathematics, 20.05.2021 23:40

Health, 20.05.2021 23:40

English, 20.05.2021 23:40

Mathematics, 20.05.2021 23:40

History, 20.05.2021 23:40

Mathematics, 20.05.2021 23:40

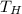 = 1200 K
= 1200 K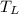 = 600 K
= 600 K