
Engineering, 20.07.2019 05:20 makeithappen60
Write down the equation for the stoichiometric combustion of ethane (c2h6) with oxygen, and determine the stoichiometric air fuel mass ratio. atomic weights: h=1, c=12, o=16 (all kg/kmol), air composition: 23.3% oxygen, 76.7% nitrogen (by mass).

Answers: 3


Another question on Engineering

Engineering, 04.07.2019 18:10
The temperature of air decreases as it is compressed by an adiabatic compressor. a)- true b)- false
Answers: 2

Engineering, 04.07.2019 18:10
Ifa component is made of two or more materials with different modulus of elasticity (e), it is called a composite member and we calculate the factor·n". mention the formula for calculating n". also, ifn> 1, explain what will happen to the 1. transformed.gi) ifn 1, what will happen to the material when transformed material when
Answers: 1

Engineering, 04.07.2019 18:20
Select any two (2) areas of applications of chain-drive. (clo4) a)-permanent lubrication necessary b)-hydraulic forklift truck operation c)-rigging and heavy moving materials d)-relatively high maintenance costs e)-costlier than belt drives
Answers: 2

Engineering, 04.07.2019 19:10
The short distance from the objective lens to the object causes problems at high magnification. which of the following is the most serious? a. cleaning the object surface b. positioning the object c. reflection from the object surface. d. illumination of the object
Answers: 1
You know the right answer?
Write down the equation for the stoichiometric combustion of ethane (c2h6) with oxygen, and determin...
Questions



History, 12.01.2022 03:40


Social Studies, 12.01.2022 03:40


SAT, 12.01.2022 03:40



Mathematics, 12.01.2022 03:50



SAT, 12.01.2022 03:50

Chemistry, 12.01.2022 03:50



Health, 12.01.2022 03:50


Mathematics, 12.01.2022 03:50

Mathematics, 12.01.2022 03:50

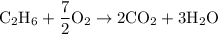
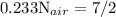
![\text{N}_{air} = 15,0 \text{kmol} [\tex]As molar weight of ethane is [tex]\dfrac{M_{air}}{M_{ethane}} = \dfrac{N_{air}\cdot MW_{air}}{N_{ethane}\cdot MW_{etane}} = \dfrac{15kmol\cdot 28.9kg/kmol}{1kmol\cdot 30kg/kmol}=14.45](/tpl/images/0110/5262/800f9.png) and air is 0.233*32 + 0.767*28 = 28.9kg/kmol, the mass ratio is:
and air is 0.233*32 + 0.767*28 = 28.9kg/kmol, the mass ratio is:

