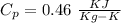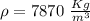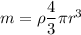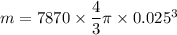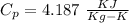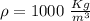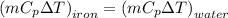Engineering, 30.08.2019 05:30 Tayj91
A5 cm diameter iron ball at 80°c is dropped into an insulated tank that contains 0.5 m^3 of liquid water at 25°c. determine the temperature when thermal equilibrium is reached.

Answers: 2


Another question on Engineering

Engineering, 03.07.2019 15:10
Apiston-cylinder with a volume of 0.25 m3 holds 1 kg of air (r 0.287 k/kgk) at a temperature of 100 c. heat transfer to the cylinder causes an isothermal expansion of the piston until the volume triples. how much heat is added to the piston-cylinder?
Answers: 3

Engineering, 04.07.2019 18:10
The temperature of air decreases as it is compressed by an adiabatic compressor. a)- true b)- false
Answers: 2

Engineering, 04.07.2019 18:10
An ideal otto cycle with air as the working fluid has a compression ratio of 8. the minimum and maximum temperatures in the cycle are 300 k and 1340 k. use constant specific heats at room temperature to determine (a) the amount of heat transferred to the air during the heat- addition kj/kg, (b) the thermal efficiency, and (c) the thermal efficiency of a carnot cycle ope limits. process, in rating between the same temperature
Answers: 2

Engineering, 04.07.2019 18:10
Condition monitoring is a major component of. (clo4) a)- predictive maintenance. b)-preventive maintenance c)-proactive maintenance d)-reactive maintenance.
Answers: 1
You know the right answer?
A5 cm diameter iron ball at 80°c is dropped into an insulated tank that contains 0.5 m^3 of liquid w...
Questions


Mathematics, 07.11.2020 19:30

Computers and Technology, 07.11.2020 19:30


Social Studies, 07.11.2020 19:30

English, 07.11.2020 19:30

Mathematics, 07.11.2020 19:30

Advanced Placement (AP), 07.11.2020 19:30



Mathematics, 07.11.2020 19:30


History, 07.11.2020 19:30



Mathematics, 07.11.2020 19:30



Mathematics, 07.11.2020 19:30

English, 07.11.2020 19:30