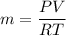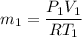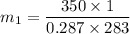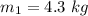Engineering, 14.11.2019 04:31 nate7742
A1-m3 tank containing air at 10°c and 350 kpa is connected through a valve to another tank containing 3 kg of air at 35°c and 150 kpa. now the valve is opened, and the entire system is allowed to reach thermal equilibrium with the surroundings, which are at 19.5°c. determine the volume of the second tank and the final equilibrium pressure of air. the gas constant of air is r = 0.287 kpa·m3/kg·k.

Answers: 2


Another question on Engineering

Engineering, 03.07.2019 23:20
Two technicians are discussing the intake air temperature (iat) sensor. technician a says that the computer uses the iat sensor as a backup to the engine coolant temperature (ect) sensor. technician b says that the powertrain control module (pcm) will subtract the calculated amount of fuel if the air measures hot. who is correct
Answers: 3

Engineering, 04.07.2019 18:10
Fluids at rest possess no flow energy. a)- true b)- false
Answers: 3

Engineering, 04.07.2019 18:10
An ideal otto cycle with air as the working fluid has a compression ratio of 8. the minimum and maximum temperatures in the cycle are 300 k and 1340 k. use constant specific heats at room temperature to determine (a) the amount of heat transferred to the air during the heat- addition kj/kg, (b) the thermal efficiency, and (c) the thermal efficiency of a carnot cycle ope limits. process, in rating between the same temperature
Answers: 2

Engineering, 04.07.2019 18:20
Ahe-xe mixture containing a 0.75 mole fraction of helium is used for cooling electronics in an avionics application. at a temperature of 300 k and atmospheric pressure, calculate the mass fraction of helium and the mass density, molar concentration and molecular weight of the mixture. if the cooling capacity is 10 l, what is the mass of the coolant?
Answers: 3
You know the right answer?
A1-m3 tank containing air at 10°c and 350 kpa is connected through a valve to another tank containin...
Questions


Social Studies, 02.02.2020 10:43

Mathematics, 02.02.2020 10:43


English, 02.02.2020 10:43

Mathematics, 02.02.2020 10:43








Biology, 02.02.2020 10:43

History, 02.02.2020 10:43



Chemistry, 02.02.2020 10:43

English, 02.02.2020 10:43