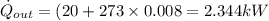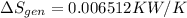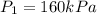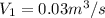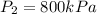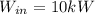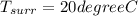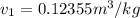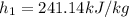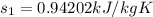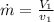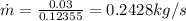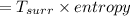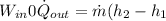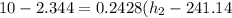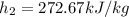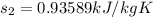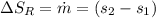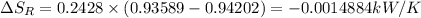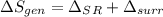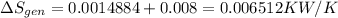Engineering, 19.12.2019 04:31 brittanysanders
Refrigerant-134a enters a compressor as a saturated vapor at 160 kpa at a rate of 0.03 m3/s and leaves at 800 kpa. the power input to the compressor is 10 kw. if the surroundings at 20°c experience an entropy increase of 0.008 kw/k, determine (a) the rate of heat loss from the compressor, (b) the exit temperature of the refrigerant, and (c) the rate of entropy generation.

Answers: 3


Another question on Engineering

Engineering, 03.07.2019 14:10
Amass of 1.5 kg of air at 120 kpa and 24°c is contained in a gas-tight, frictionless piston-cylinder device. the air is now compressed to a final pressure of 720 kpa. during the process, heat is transferred from the air such that the temperature inside the cylinder remains constant. calculate the boundary work input during this process.
Answers: 2

Engineering, 04.07.2019 18:10
Atmospheric air has a temperature (dry bulb) of 80° f and a wet bulb temperature of 60° f when the barometric pressure is 14.696 psia. determine the specific humidity, grains/lb dry air. a. 11.4 c. 55.8 d. 22.5 b. 44.1
Answers: 1

Engineering, 04.07.2019 18:20
Find the kinematic pressure of 160kpa. for air, r-287 j/ kg k. and hair al viscosity of air at a temperature of 50°c and an absolute (10 points) (b) find the dynamic viscosity of air at 110 °c. sutherland constant for air is 111k
Answers: 3

Engineering, 04.07.2019 18:20
Asimple rankine cycle uses water as the working fluid. the water enters the turbine at 10 mpa and 480c while the condenser operates at 6 kpa. if the turbine has an isentropic efficiency of 80 percent while the pump has an isentropic efficiency of 70 percent determine the thermal efficiency
Answers: 1
You know the right answer?
Refrigerant-134a enters a compressor as a saturated vapor at 160 kpa at a rate of 0.03 m3/s and leav...
Questions

Mathematics, 26.02.2022 16:50



Mathematics, 26.02.2022 16:50

English, 26.02.2022 16:50

English, 26.02.2022 16:50




Mathematics, 26.02.2022 16:50

SAT, 26.02.2022 16:50

Mathematics, 26.02.2022 16:50



History, 26.02.2022 16:50



Business, 26.02.2022 17:00

Mathematics, 26.02.2022 17:00