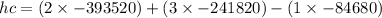Engineering, 28.12.2019 06:31 bravoy
Ethane (c2h6) is burned at atmospheric pressure with a stoichiometric amount of air as the oxidizer. determine the heat rejected, in kj/kmol fuel, when the products and reactants are both at 25c, and the water vapor appears in the products as water vapor. ( 1,427,820 kj/kmol)

Answers: 3


Another question on Engineering

Engineering, 03.07.2019 15:10
Apiston-cylinder with a volume of 0.25 m3 holds 1 kg of air (r 0.287 k/kgk) at a temperature of 100 c. heat transfer to the cylinder causes an isothermal expansion of the piston until the volume triples. how much heat is added to the piston-cylinder?
Answers: 3

Engineering, 04.07.2019 18:10
Items are similar to the free issue items, but their access is limited. (clo5) a)-bin stock items free issue b)-bin stock controlled issue c)-critical or insurance spares d)-rebuildable spares e)-consumables
Answers: 1

Engineering, 06.07.2019 04:20
Derive the 2-d finite difference equation for a uniform grid for a node: a) at a plane surface with a uniform heat flux b) at an internal corner with convection
Answers: 1

Engineering, 06.07.2019 04:30
Asharp-edged orifice is placed in a 10-in-diameter pipe carrying ammonia. if the volume flow rate is 25 gal/min, calculate the deflection of a water manometer (a) if the orifice diameter is 1.0 in and (b) if the orifice diameter is 7.0 in. the ammonia has a specific gravity of 0.83 and a dynamic viscosity of 2.5 x 10-6 lb s/t2
Answers: 3
You know the right answer?
Ethane (c2h6) is burned at atmospheric pressure with a stoichiometric amount of air as the oxidizer....
Questions


Mathematics, 05.02.2021 04:50




Mathematics, 05.02.2021 04:50

Spanish, 05.02.2021 04:50

Mathematics, 05.02.2021 04:50


Mathematics, 05.02.2021 04:50

Business, 05.02.2021 04:50


Mathematics, 05.02.2021 04:50


Mathematics, 05.02.2021 04:50

History, 05.02.2021 04:50


History, 05.02.2021 04:50

Physics, 05.02.2021 04:50

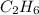
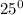
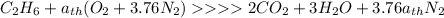
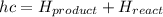
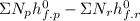
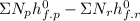
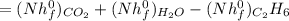
![h^{0} _{f} = enthalpy of formation at the standard reference stateFrom the enthalpy of formation tables at 25 degrees and 1 atmTaking enathalpy of formation of [tex]CO_{2}](/tpl/images/0435/5462/b912a.png) = -393520 KJ/mol
= -393520 KJ/mol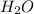 = -241820 KJ/mol
= -241820 KJ/mol