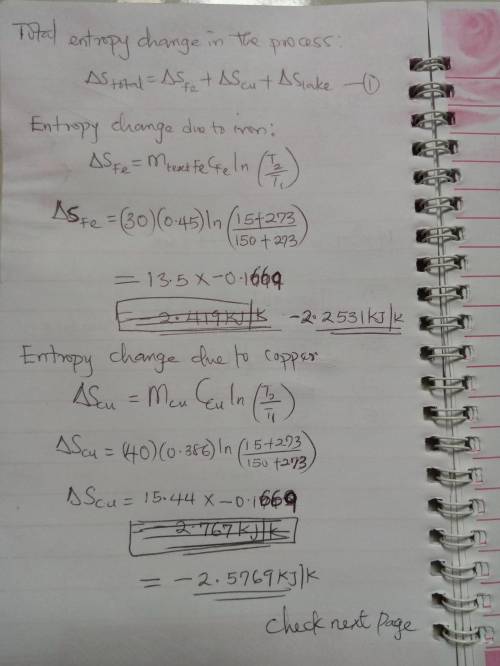Engineering, 18.02.2020 05:25 dewayne5599
A 30-kg iron block and a 40-kg copper block, both initially at 150oC, are dropped into a large lake at 15oC. Thermal equilibrium is established after a while as a result of heat transfer between the blocks and the lake water. Determine the total entropy change for this process.

Answers: 3


Another question on Engineering

Engineering, 04.07.2019 18:10
At 12 noon, the count in a bacteria culture was 400; at 4: 00 pm the count was 1200 let p(t) denote the bacteria cou population growth law. find: (a) an expression for the bacteria count at any time t (b) the bacteria count at 10 am. (c) the time required for the bacteria count to reach 1800.
Answers: 1

Engineering, 04.07.2019 19:10
When subject to a steady load (within elastic range) over a long period of time, what is the major difference in material behavoir between steel and plastic?
Answers: 2

Engineering, 06.07.2019 03:30
Acubic shaped box has a side length of 1.0 ft and a mass of 10 lbm is sliding on a frictionless horizontal surface towards a 30 upward incline. the horizontal velocity of the box is 20 ft/s. determine how far up the incline the box will travel (report center of mass distance along the inclined surface, not vertical distance)
Answers: 1

Engineering, 06.07.2019 04:10
An inventor claims to have developed a reversed heat engine (ie. a heat pump) which is able to deliver 10 kj heat into the room at 20°c from outside ambient temperature of -10°c by consuming only 2 kj electricity in winter. this claim is a)-impossible b)-possible only if the heat pump is ideal c)-practically possible d)-unable to be assessed as it depends on the working fluid used in the heat pump.
Answers: 2
You know the right answer?
A 30-kg iron block and a 40-kg copper block, both initially at 150oC, are dropped into a large lake...
Questions


History, 10.02.2021 20:10



Mathematics, 10.02.2021 20:10

Biology, 10.02.2021 20:10


Mathematics, 10.02.2021 20:10

Mathematics, 10.02.2021 20:10

Social Studies, 10.02.2021 20:10

Mathematics, 10.02.2021 20:10


History, 10.02.2021 20:10

Social Studies, 10.02.2021 20:10

Mathematics, 10.02.2021 20:10



Mathematics, 10.02.2021 20:10


Mathematics, 10.02.2021 20:10