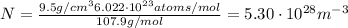Engineering, 20.02.2020 04:31 oof40
Calculate the energy for vacancy formation in silver, given that the equilibrium number of vacanciesat 800°C (1073 K) is 3.6 × 1023m–3. The atomic weight and density (at 800°C) for silver are, respectively, 107.9 g/mol and 9.5 g/cm3. (hint: see example problem in Lecture 9for part of the solution)

Answers: 2


Another question on Engineering

Engineering, 03.07.2019 15:10
Apiston-cylinder with a volume of 0.25 m3 holds 1 kg of air (r 0.287 k/kgk) at a temperature of 100 c. heat transfer to the cylinder causes an isothermal expansion of the piston until the volume triples. how much heat is added to the piston-cylinder?
Answers: 3

Engineering, 04.07.2019 18:20
Modern high speed trains do not have perpendicular expansion gaps where rails are joined end-to-end any more they are mostly welded together but what might happen if there was a spell of particularly hot weather that causes inspection of the tracks?
Answers: 1

Engineering, 04.07.2019 19:10
How to increase the thermal officiency of an ideal simple rankino cycle? among these methods, which one is the best and why?
Answers: 2

Engineering, 04.07.2019 19:10
A)-explain briefly the importance of standards in engineering design. b)- what is patent? c)-explain the relationship between these standards: b.s. and b.s.en d)- in engineering design concepts, types of loads and how they act are important factors. explain.
Answers: 3
You know the right answer?
Calculate the energy for vacancy formation in silver, given that the equilibrium number of vacancies...
Questions




Mathematics, 24.04.2020 00:00

Biology, 24.04.2020 00:00






English, 24.04.2020 00:00


Mathematics, 24.04.2020 00:00

History, 24.04.2020 00:00





Mathematics, 24.04.2020 00:00

Mathematics, 24.04.2020 00:00


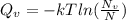 (1)
(1) 
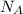 : is the Avogadro constant = 6.022x10²³ atoms/mol, and A: is the atomic weight = 107.9 g/mol.
: is the Avogadro constant = 6.022x10²³ atoms/mol, and A: is the atomic weight = 107.9 g/mol.