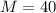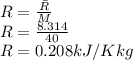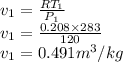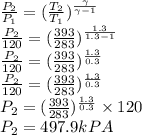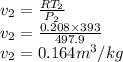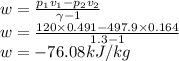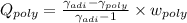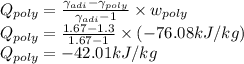Engineering, 21.02.2020 02:36 queenjade2614
Argon is compressed in a polytropic process with = 1:3 (i. e., PV = costant) from 120 kPa and 10 C until it reaches a temperature of 120 C in a piston{cylinder device. Model the system as an ideal gas and determine the speci c work (work per unit mass) done by the gas and the heat per unit mass transferred during this the process.

Answers: 1


Another question on Engineering

Engineering, 03.07.2019 15:10
Apiston-cylinder with a volume of 0.25 m3 holds 1 kg of air (r 0.287 k/kgk) at a temperature of 100 c. heat transfer to the cylinder causes an isothermal expansion of the piston until the volume triples. how much heat is added to the piston-cylinder?
Answers: 3

Engineering, 04.07.2019 19:20
Determine the time of the day and month of the year at which the peak sensible cooling load occurs for a top floor, north-eastern corner room of an office building in durban for the following conditions: floor area: 8 x 8 x 3 m east and north walls: 115 mm face brick (outside), 20 mm air space, 115 mm ordinary brick (inside) with 15 mm plaster roof construction: suspended plasterboard ceiling, 450 mm air space, 150 mm concrete, 75mm screed, waterproofing no heat transfer across other surfaces window area 3x 1,5 m high in north wall only. ordinary glass with venetian blinds. lights and occupants : from 07: 00 to 18: 00 average light density: 25 w/m2 number of occupants : 5 seated, light office work room temperature 24°c
Answers: 3

Engineering, 06.07.2019 03:10
Steel balls 12 mm in diameter are annealed by heating to 1150 k and then slowly cooled to 400 k in an air att 325 k and h -20 w/m2 k. assume the properties of the steel to be k- 40 w/m k,p 7800 kg/m3, and cp 600 j/kg k. a. determine whether lumped system method can be used to analyze this problem and if so why" b. determine the time required for the cooling process. c. determine the total amount of heat lost from each ball to the ambient air
Answers: 1

Engineering, 06.07.2019 03:10
An electrical kettle is made out of stainless steel,weighs two pounds (when empty) with a heating element that consumes 2 kw of electricity. assuming that the water and the kettle are at the same uniform temperature at any moment of time, calculate the shortest possible time to bring 2 quarts of water from room temperature to the onset of boiling.
Answers: 1
You know the right answer?
Argon is compressed in a polytropic process with = 1:3 (i. e., PV = costant) from 120 kPa and 10 C u...
Questions

Arts, 18.10.2019 17:50


Social Studies, 18.10.2019 17:50

Mathematics, 18.10.2019 17:50


Mathematics, 18.10.2019 17:50

History, 18.10.2019 17:50


Computers and Technology, 18.10.2019 17:50




History, 18.10.2019 17:50


Mathematics, 18.10.2019 17:50


Mathematics, 18.10.2019 17:50

Mathematics, 18.10.2019 17:50

Biology, 18.10.2019 17:50