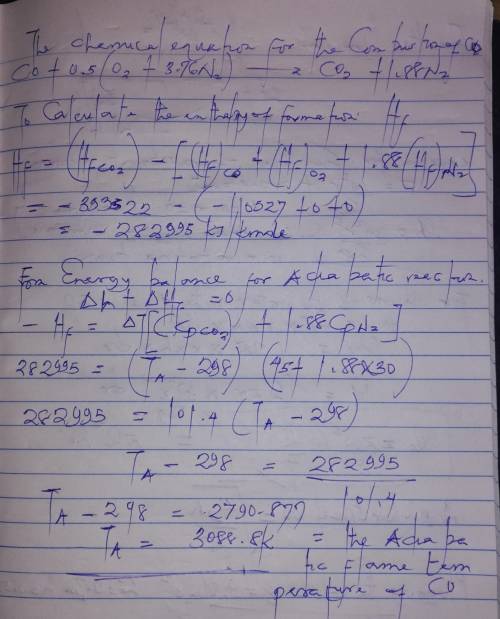Engineering, 21.02.2020 20:53 shaydog6353
Determine the adiabatic flame temperature of carbon monoxide (CO) burning in air at an equivalence ratio of unity. The reactants are at standard temperature and pressure. Assume constant specific heats.

Answers: 3


Another question on Engineering

Engineering, 03.07.2019 14:10
Explain the difference laminar and turbulent flow. explain it with the shear stress and the velocity profiles.
Answers: 1

Engineering, 03.07.2019 14:10
Line joining liquid phase with liquid and solid phase mixture is known as: a) liquidus b) solidus c) tie line d) none of the mentioned
Answers: 2

Engineering, 04.07.2019 18:10
Acompressor receives the shaft work to decrease the pressure of the fluid. a)- true b)- false
Answers: 3

Engineering, 04.07.2019 18:20
Agas mixture consists of 8 kmol of h2 and 2 kmol of n2. determine the mass of each gas and the apparent gas constant of the mixture.
Answers: 3
You know the right answer?
Determine the adiabatic flame temperature of carbon monoxide (CO) burning in air at an equivalence r...
Questions


Biology, 04.03.2020 08:12



Mathematics, 04.03.2020 08:12


Business, 04.03.2020 08:12



English, 04.03.2020 08:12

Business, 04.03.2020 08:12

Biology, 04.03.2020 08:13






Arts, 04.03.2020 08:13

History, 04.03.2020 08:14

English, 04.03.2020 08:14