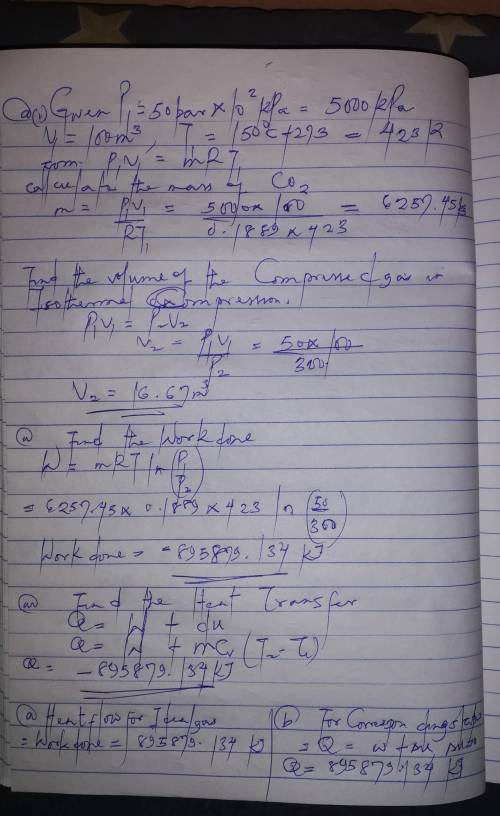Engineering, 07.03.2020 05:56 Schoolwork100
100 cubic meters of carbon dioxide initially at 150 C and 50bar is to be isothermally compressed in a frictionless piston and cylinder device to a final pressure of 300 bar. Calculate:
i. The volume of the compressed gas
ii. The work done to compress the gas
iii. The heat flow on compression assuming carbon dioxide:
a/ Is an ideal gas
b/ Obeys the principle of corresponding states
c/ Obeys the Peng-RObinson equation of state

Answers: 3


Another question on Engineering

Engineering, 04.07.2019 03:10
What precautions should you take to prevent injuries when dealing with heavy loads?
Answers: 1

Engineering, 04.07.2019 18:10
An air conditioning system consist of a 5 cm diameter pipe, operating at a pressure of 200 kpa. the air initially enters the pipe at 15°c with a velocity of 20 m/s and relative humidity of 80%. if the heat supply throughout the process is 960 w, determine the relative humidity and the temperature at the outlet
Answers: 3

Engineering, 04.07.2019 18:20
Most leaks in reciprocating air compressors can be detected and minimized by: (clo4) a)-detecting leakage areas using ultrasonic acoustic detector. b)-tightening joints and connections c)-replacing faulty equipment d)-all of the given options
Answers: 2

Engineering, 04.07.2019 18:20
Steam enters a converging nozzle at 3.0 mpa and 500°c with a at 1.8 mpa. for a nozzle exit area of 32 cm2, determine the exit velocity, mass flow rate, and exit mach number if the nozzle: negligible velocity, and it exits (a) is isentropic (b) has an efficiency of 94 percent
Answers: 2
You know the right answer?
100 cubic meters of carbon dioxide initially at 150 C and 50bar is to be isothermally compressed in...
Questions

Mathematics, 09.02.2021 19:50

Chemistry, 09.02.2021 19:50

Chemistry, 09.02.2021 19:50

Mathematics, 09.02.2021 19:50

Mathematics, 09.02.2021 19:50

History, 09.02.2021 19:50


Mathematics, 09.02.2021 19:50


Mathematics, 09.02.2021 19:50

Chemistry, 09.02.2021 19:50


Mathematics, 09.02.2021 19:50

Mathematics, 09.02.2021 19:50


Mathematics, 09.02.2021 19:50




Arts, 09.02.2021 20:00