
Engineering, 11.03.2020 22:06 elijaahstepp041
8–21 Heat in the amount of 100 kJ is transferred directly from a hot reservoir at 1200 K to a cold reservoir at 600 K. Calculate the entropy change of the two reservoirs and determine if the increase of entropy principle is satisfied

Answers: 3


Another question on Engineering

Engineering, 04.07.2019 16:10
The force on a cutting tool are 2600n vertically downward and 2100 horizontal. determine the resultant force acting on the tool and the angle at which it acts.
Answers: 1

Engineering, 04.07.2019 18:10
For the closed feedwater heater below, feedwater enters state 3 at a pressure of 2000 psia and temperature of 420 °f at a rate of ix10 ibhr. the feedwat extracted steam enters state 1 at a pressure of 1000 psia and enthalpy of 1500 btu/lbm. the extracted er leaves at an enthalpy of 528.7 btu/lbm steam leaves as a saturated liquid. (16) a) determine the mass flow rate of the extraction steam used to heat the feedwater (10) b) determine the terminal temperature difference of the closed feedwater heater
Answers: 3

Engineering, 04.07.2019 18:10
Calculate the bore of a cylinder that has a stroke of 18 inches and an extension time of 6 seconds at a flow rate of 4 gal/min.
Answers: 3

Engineering, 04.07.2019 18:10
Ahot wire operates at a temperature of 200°c while the air temperature is 20°c. the hot wire element is a tungsten wire of 5 um diameter and 2 mm in length. plot using excel current, heat transfer and heat generated by the wire for air velocity varying from 1-10 m/s in steps of lm/s? matlab the sensor voltage output, resistance, or assume nu 0.989 re033pr13 take air properties at tr (200°c20°c)/2 = 110°c properties of tungsten: c 0.13 kj/kg.k 3 p 19250 kg/m k (thermal conductivity) = 174 w/m.k
Answers: 2
You know the right answer?
8–21 Heat in the amount of 100 kJ is transferred directly from a hot reservoir at 1200 K to a cold r...
Questions

English, 18.05.2021 16:50


Mathematics, 18.05.2021 16:50


Mathematics, 18.05.2021 16:50



Mathematics, 18.05.2021 16:50

Chemistry, 18.05.2021 16:50

English, 18.05.2021 16:50



Mathematics, 18.05.2021 16:50




English, 18.05.2021 16:50

Computers and Technology, 18.05.2021 16:50

Spanish, 18.05.2021 16:50

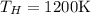
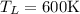
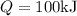
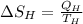
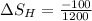
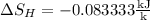
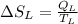
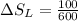
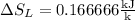
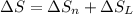
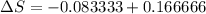
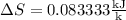
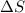 is not less than zero.Hence,it fulfills increase of entropy principle.
is not less than zero.Hence,it fulfills increase of entropy principle. 

