
Engineering, 11.03.2020 22:01 aliyahmuhammad5197
A piston-cylinder device initially contains 0.5m3 of nitrogen gas at 400 kPa and 27 °C. Anelectric heater within the device is turned on and is allowed to pass a current of 2 A for 5min from a 120V source. Nitrogen expands at constant pressure, and a heat loss of 2800 Joccurs during the process. Determine the final temperature of nitrogen. Assume nitrogen to be an ideal gas with M = 28 g/mol and cp=1.039 kJ/kg. K

Answers: 1


Another question on Engineering

Engineering, 03.07.2019 15:10
Apiston-cylinder with a volume of 0.25 m3 holds 1 kg of air (r 0.287 k/kgk) at a temperature of 100 c. heat transfer to the cylinder causes an isothermal expansion of the piston until the volume triples. how much heat is added to the piston-cylinder?
Answers: 3

Engineering, 04.07.2019 18:10
The temperature of air decreases as it is compressed by an adiabatic compressor. a)- true b)- false
Answers: 2

Engineering, 04.07.2019 18:10
The filament of an incandescent lamp has a temperature of 2000k. calculate the fraction of radiation emitted in the visible light band if the filament is approximated as blackbody
Answers: 2

Engineering, 04.07.2019 18:10
Condition monitoring is a major component of. (clo4) a)- predictive maintenance. b)-preventive maintenance c)-proactive maintenance d)-reactive maintenance.
Answers: 1
You know the right answer?
A piston-cylinder device initially contains 0.5m3 of nitrogen gas at 400 kPa and 27 °C. Anelectric h...
Questions

Mathematics, 16.10.2019 12:00




Physics, 16.10.2019 12:10


English, 16.10.2019 12:10

Biology, 16.10.2019 12:10

Mathematics, 16.10.2019 12:10




Health, 16.10.2019 12:10

Mathematics, 16.10.2019 12:10


Mathematics, 16.10.2019 12:10

Biology, 16.10.2019 12:10

Mathematics, 16.10.2019 12:10


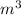
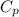 = 1.039 KJ/Kg K
= 1.039 KJ/Kg K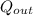 = 2800
= 2800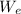 ) = VI Δt
) = VI Δt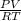
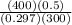
 -
- 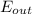 = ΔE system
= ΔE system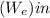 -
- 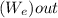 = ΔU
= ΔU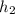 -
- 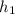 )
)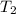 -
-  )
)

