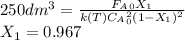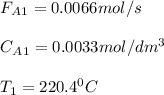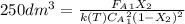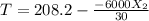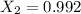Engineering, 21.03.2020 10:21 Harms
The elementary irreversible organic liquid-phase reaction A+B --> C is carried out adiabatically in a flow reactor. An equal molar feed of A and B enters at 27oC the reactor. The volumetric flow rate is 2 dm3 /s and CA0 = 0.1kmol/m3
a.) Calculate the PFR and CSTR volumes necessary to achieve 85% conversion.
b.) What is the maximum inlet temperature on could have so that the boiling point of the liquid (550K) would not be exceeded even for complete conversion?
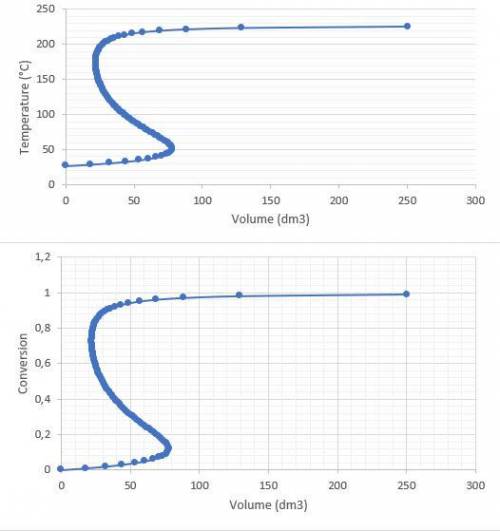
c.) Plot the conversion and temperature as a function of PFR volume (i. e., distance down the reactor)
d.) Calculate the conversion that can be achieved in one 500dm3 CSTR and in two 250dm3 CSTRs in series.
Additional information:
A B C
H (273K) (kcal/mol) -20 -15 -41
Cp (cal/mol*K) 15 15 30
k=0.01 dm3 /mol. s at 300K, E=10,000 cal/mol

Answers: 1


Another question on Engineering

Engineering, 03.07.2019 15:10
Heat is added to a piston-cylinder device filled with 2 kg of air to raise its temperature 400 c from an initial temperature of t1 27 cand pressure of pi 1 mpa. the process is isobaric process. find a)-the final pressure p2 b)-the heat transfer to the air.
Answers: 1

Engineering, 04.07.2019 18:10
Ahot wire operates at a temperature of 200°c while the air temperature is 20°c. the hot wire element is a tungsten wire of 5 um diameter and 2 mm in length. plot using excel current, heat transfer and heat generated by the wire for air velocity varying from 1-10 m/s in steps of lm/s? matlab the sensor voltage output, resistance, or assume nu 0.989 re033pr13 take air properties at tr (200°c20°c)/2 = 110°c properties of tungsten: c 0.13 kj/kg.k 3 p 19250 kg/m k (thermal conductivity) = 174 w/m.k
Answers: 2

Engineering, 04.07.2019 18:20
For each of the following process: a) sketch the p-v diagram, b)sketch t-s diagram, c) sketch t-v diagram, d) sketch the boundary work on one of the diagrams (a, b or c) and e) sketch the reversible heat transfer on one of the diagrams (a, b or c): 1- isobaric process from compressed liquid to superheated vapor 2- isothermal process from compressed liquid to superheated vapor 3- isentropic process from compressed liquid to superheated vapor
Answers: 3

Engineering, 04.07.2019 19:10
10 kg of co2 is initially contained at 400 kpa and 300 k. the gas constant for carbon dioxide is 189 j/lkg k) and has a specific heat ratio, k, of 1.289. isentropic expansion then occurs until the pressure is 200 kpa. a) determine the initial volume of co2 in m. b) determine the final temperature in k. c) determine the work done by the system during the expansion kl.
Answers: 2
You know the right answer?
The elementary irreversible organic liquid-phase reaction A+B --> C is carried out adiabatically...
Questions

Social Studies, 12.05.2020 02:57


English, 12.05.2020 02:57

English, 12.05.2020 02:57




Health, 12.05.2020 03:57

Mathematics, 12.05.2020 03:57

Mathematics, 12.05.2020 03:57

Mathematics, 12.05.2020 03:57

Mathematics, 12.05.2020 03:57



Mathematics, 12.05.2020 03:57





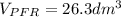 and
and 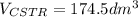
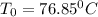
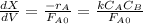
![\frac{dX}{dV}=[k_{(300K)}*exp(\frac{E}{R}(\frac{1}{300}-\frac{1}{T})]}\frac{C_AC_B}{F_A_0}](/tpl/images/0557/6585/95b8a.png)
![\frac{dX}{dV}=[k_{(300K)}*exp(\frac{E}{R}(\frac{1}{300}-\frac{1}{T})]}\frac{C_A_0^2}{F_A_0}(1-X)^2](/tpl/images/0557/6585/791bb.png)
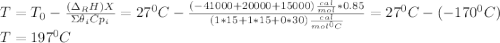
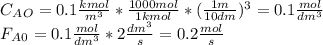
![\int\limits^{0.85}_0 {\frac{1}{(1-X)^2} } \, dX =[0.01dm^3/(mol*s)*exp(\frac{10000cal/mol}{ 1.9872cal/mol*K}(\frac{1}{300K}-\frac{1}{(197+273.15)K})]}\frac{(0.1mol/dm^3)^2}{0.2mol/s}\int\limits^V_0 {} \, dV \\\\5.667=0.216dm^{-3}V\\V=5.667/0.216dm^{-3}\\V_{PFR}=26.3dm^{3}](/tpl/images/0557/6585/b748f.png) Now, for the CSTR, just the design equation is changed by:
Now, for the CSTR, just the design equation is changed by: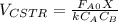
![V_{CSTR}=\frac{0.2mol/s*0.85}{[0.01dm^3/(mol*s)*exp(\frac{10000cal/mol}{ 1.9872cal/mol*K}(\frac{1}{300K}-\frac{1}{(197+273.15)K})]*(0.1mol/dm^3)^2(1-0.85)^2} \\V_{CSTR}=\frac{0.17mol/s}{4.33dm^3/(mol*s)*0.01mol^2/dm^6(0.0225)} \\V_{CSTR}=174.5dm^3](/tpl/images/0557/6585/090b3.png)
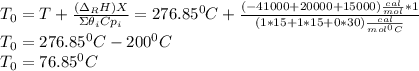
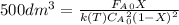
![k(T)=[0.01dm^3/(mol*s)*exp(\frac{10000cal/mol}{1.9872cal/mol*K}(\frac{1}{300K}-\frac{1}{(T+273.15)K})]](/tpl/images/0557/6585/8e617.png)