
Engineering, 30.03.2020 20:30 zitterkoph
A piston–cylinder device contains a mixture of 0.5 kg of H2 and 1.2 kg of N2 at 100 kPa and 300 K. Heat is now transferred to the mixture at constant pressure until the volume is doubled. Assuming constant specific heats at the average temperature, determine (a) the heat transfer and (b) the entropy change of the mixture. 13–68E During the expansion process of the ideal Otto

Answers: 2


Another question on Engineering

Engineering, 04.07.2019 18:10
Coiled springs ought to be very strong and stiff. si3n4 is a strong, stiff material. would you select this material for a spring? explain.
Answers: 2

Engineering, 04.07.2019 18:10
Different types of steels contain different elements that alter the characteristics of the steel. for each of the following elements, explain what the element does when alloyed with steel.
Answers: 2

Engineering, 04.07.2019 18:10
Thermal stresses are developed in a metal when its a) initial temperature is changed b) final temperature is changed c) density is changed d) thermal deformation is prevented e) expansion is prevented f) contraction is prevented
Answers: 2

Engineering, 04.07.2019 18:10
At 12 noon, the count in a bacteria culture was 400; at 4: 00 pm the count was 1200 let p(t) denote the bacteria cou population growth law. find: (a) an expression for the bacteria count at any time t (b) the bacteria count at 10 am. (c) the time required for the bacteria count to reach 1800.
Answers: 1
You know the right answer?
A piston–cylinder device contains a mixture of 0.5 kg of H2 and 1.2 kg of N2 at 100 kPa and 300 K. H...
Questions

Mathematics, 12.12.2020 16:10

Mathematics, 12.12.2020 16:10

Computers and Technology, 12.12.2020 16:10


Chemistry, 12.12.2020 16:10





Mathematics, 12.12.2020 16:10

Mathematics, 12.12.2020 16:10

History, 12.12.2020 16:10

Mathematics, 12.12.2020 16:10

English, 12.12.2020 16:10

Mathematics, 12.12.2020 16:10

Mathematics, 12.12.2020 16:10


Mathematics, 12.12.2020 16:10


History, 12.12.2020 16:10

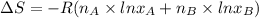
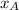 and
and 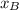 are the mole fractions of Hydrogen and nitrogen respectively.
are the mole fractions of Hydrogen and nitrogen respectively.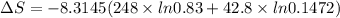 = 1066.0279 J/K
= 1066.0279 J/K

