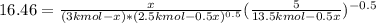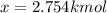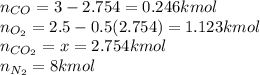Engineering, 02.04.2020 03:10 maiahfogel1351
An equilibrium mixture of 3 kmol of CO, 2.5 kmol of O2, and 8 kmol of N2 is heated to 2600 K at a pressure of 5 atm. Determine the equilibrium composition of the mixture for these conditions.

Answers: 1


Another question on Engineering

Engineering, 03.07.2019 14:10
Line joining liquid phase with liquid and solid phase mixture is known as: a) liquidus b) solidus c) tie line d) none of the mentioned
Answers: 2

Engineering, 04.07.2019 12:10
On a average work day more than work place firs are reorted
Answers: 1

Engineering, 04.07.2019 18:10
During a steady flow process, the change of energy with respect to time is zero. a)- true b)- false
Answers: 2

Engineering, 04.07.2019 18:10
Apipe with an outside diameter of 15 cm is exposed to an ambient air and surrounding temperature of -20°c. the pipe has an outer surface temperature of 65°c and an emissivity of 0.85. if the rate of heat loss from the pipe surface is 0.95 kw per meter of length, the external convective heat transfer coefficient (h) is: (a) 12.5 w/m"k (b) 18.6 w/mk (c) 23.7 w/mk (d) 27.9 w/mk (e) 33.5 w/mk
Answers: 1
You know the right answer?
An equilibrium mixture of 3 kmol of CO, 2.5 kmol of O2, and 8 kmol of N2 is heated to 2600 K at a pr...
Questions

Mathematics, 19.04.2021 17:30



Mathematics, 19.04.2021 17:30


Chemistry, 19.04.2021 17:30




Health, 19.04.2021 17:30

Mathematics, 19.04.2021 17:30

English, 19.04.2021 17:30


Mathematics, 19.04.2021 17:30




Biology, 19.04.2021 17:30

Mathematics, 19.04.2021 17:30

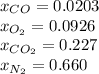
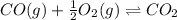
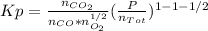
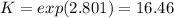
 the equilibrium goes:
the equilibrium goes: