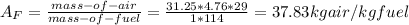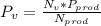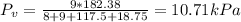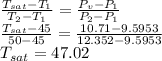Engineering, 06.04.2020 15:19 lukeritsema88
Octane (C8H18) is burned with 250 percent theoretical air, which enters the combustion chamber at 25°C. Assume complete combustion and a total pressure of 1.8 atm. determine (a) the air-fuel ratio and (b) the dew-point temperature of the products.

Answers: 3


Another question on Engineering

Engineering, 03.07.2019 14:10
The y form of iron is known as: a) ferrite b) cementite c) perlite d) austenite
Answers: 3

Engineering, 04.07.2019 18:10
Ifa component is made of two or more materials with different modulus of elasticity (e), it is called a composite member and we calculate the factor·n". mention the formula for calculating n". also, ifn> 1, explain what will happen to the 1. transformed.gi) ifn 1, what will happen to the material when transformed material when
Answers: 1

Engineering, 04.07.2019 18:20
Agas mixture consists of 8 kmol of h2 and 2 kmol of n2. determine the mass of each gas and the apparent gas constant of the mixture.
Answers: 3

Engineering, 04.07.2019 18:20
For a gate width of 2 m into the paper, determine the force required to hold the gate abc at its location.
Answers: 1
You know the right answer?
Octane (C8H18) is burned with 250 percent theoretical air, which enters the combustion chamber at 25...
Questions


Physics, 18.10.2020 14:01


English, 18.10.2020 14:01

Social Studies, 18.10.2020 14:01


Mathematics, 18.10.2020 14:01


Mathematics, 18.10.2020 14:01




English, 18.10.2020 14:01



Physics, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01

SAT, 18.10.2020 14:01


Geography, 18.10.2020 14:01