
Engineering, 07.04.2020 19:36 miajacobs110
For some transformation having kinetics that obey the Avrami equation, the parameter n is known to have a value of 1.7. If, after 100 s, the reaction is 50% complete, how long (total time) will it take the transformation to go to 99% completion?

Answers: 3


Another question on Engineering

Engineering, 03.07.2019 15:10
Apiston-cylinder with a volume of 0.25 m3 holds 1 kg of air (r 0.287 k/kgk) at a temperature of 100 c. heat transfer to the cylinder causes an isothermal expansion of the piston until the volume triples. how much heat is added to the piston-cylinder?
Answers: 3

Engineering, 04.07.2019 18:10
Carbon dioxide gas expands isotherm a turbine from 1 mpa, 500 k at 200 kpa. assuming the ideal gas model and neglecting the kinetic and potential energies, determine the change in entropy, heat transfer and work for each kilogram of co2.
Answers: 2

Engineering, 04.07.2019 18:10
You are making beer. the first step is filling the glass carboy with the liquid wort. the internal diameter of the carboy is 15 in., and you wish to fill it up to a depth of 2 ft. if your wort is drawn from the kettle using a siphon process that flows at 3 gpm, how long will it take to fill?
Answers: 1

Engineering, 04.07.2019 19:10
A)-in the process of engineering design, explain the contribution of material selection. b)- explain the procedure of synthesis as is employed in engineering design. c)- is there any relationship between ergonomics and engineering design? explain. d)- safety consideration in engineering design includes human, product and the enviroment . explain how safety will be incorporated into the design?
Answers: 3
You know the right answer?
For some transformation having kinetics that obey the Avrami equation, the parameter n is known to h...
Questions

English, 29.06.2019 18:00



World Languages, 29.06.2019 18:00

Mathematics, 29.06.2019 18:00

Computers and Technology, 29.06.2019 18:00

Geography, 29.06.2019 18:00


Mathematics, 29.06.2019 18:00


English, 29.06.2019 18:00




Arts, 29.06.2019 18:00

History, 29.06.2019 18:00

History, 29.06.2019 18:00



Biology, 29.06.2019 18:00

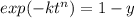
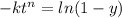
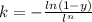
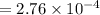
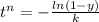
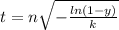
![= \sqrt[1.7]{-\frac{ln(1-0.99)}{2.76\times 10^{-4}} }](/tpl/images/0586/9814/555de.png)
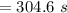
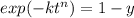
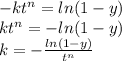
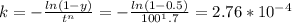
![t^n=-\frac{ln(1-y)}{k}\\t=\sqrt[n]{-\frac{ln(1-y)}{k}}](/tpl/images/0586/9814/cb2eb.png)
![t=\sqrt[n]{-\frac{ln(1-y)}{k}}=\sqrt[1.7]{-\frac{ln(1-0.99)}{2.76*10^{-4}}}=304.6s](/tpl/images/0586/9814/e1aa1.png)


