Engineering, 05.05.2020 19:58 vixen0528ow6mza
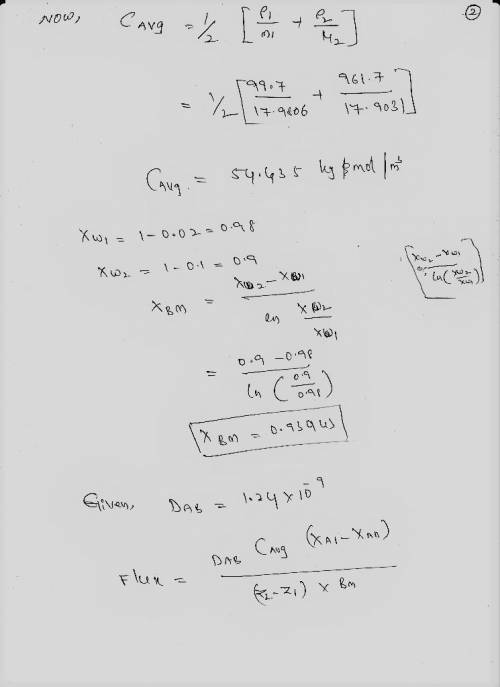
Diffusion of Ammonia in an Aqueous Solution Ammonia (A)-water (B) solution ta 278 K and 4 mm thick is in contact with an organic liquid at this interface. The concentration of ammonia in the organic phase is held constant and is such that the equilibrium concentration of ammonia at the interface is 2.0 wt% ammonia (density of aqueous solution is 991.7 Kg/m) The concentration of ammonia in water 4 mm away is 10 wt% (density of solution is 961.7 Kg/m). Water and the organic phase are insoluble in each other. The diffusion coefficient of NH3 in water is 1.24 × 10-9 m2/s.
(a) At steady state, calculate Na in Kmol/m-s
(b) Calculate NB. Explain.

Answers: 2


Another question on Engineering

Engineering, 04.07.2019 16:10
The force on a cutting tool are 2600n vertically downward and 2100 horizontal. determine the resultant force acting on the tool and the angle at which it acts.
Answers: 1

Engineering, 04.07.2019 18:10
Acompressor receives the shaft work to decrease the pressure of the fluid. a)- true b)- false
Answers: 3

Engineering, 04.07.2019 18:10
Courses that are developed by subject matter experts, internal or extemal to the college or university. these programs are marketed by the school (clo2) marks a)-vocational schools b)-vendor training c)-colleges & universities d)-continuing education programs
Answers: 2

Engineering, 04.07.2019 19:10
What are the major differences between injection molding and extrusion?
Answers: 2
You know the right answer?
Diffusion of Ammonia in an Aqueous Solution Ammonia (A)-water (B) solution ta 278 K and 4 mm thick i...
Questions



English, 03.03.2021 14:40



Mathematics, 03.03.2021 14:40




SAT, 03.03.2021 14:40

Health, 03.03.2021 14:40

Mathematics, 03.03.2021 14:40

Mathematics, 03.03.2021 14:40



Mathematics, 03.03.2021 14:40

Business, 03.03.2021 14:40


History, 03.03.2021 14:40

Spanish, 03.03.2021 14:40