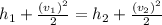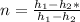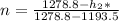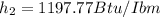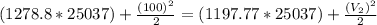Engineering, 07.05.2020 14:01 timozy95
Water vapor at 100 psi, 500 F and a velocity of 100 ft./sec enters a nozzle operating at steady sate and expands adiabatically to the exit, where the pressure is 40 psia. If the isentropic nozzle efficiency is 95%, determine for the nozzle,
(a) the exit velocity of the steam in ft./sec, and
(b) the amount of entropy produced in BTU/ lbm R.

Answers: 1


Another question on Engineering

Engineering, 04.07.2019 18:10
Fluids at rest possess no flow energy. a)- true b)- false
Answers: 3

Engineering, 04.07.2019 18:20
Apiston-cylinder device contains 0.1 m3 of liquid water and 0.9 m3 of water vapor in equilibrium at 800 kpa. heat is transferred at constant pressure until the temperature of water reaches 350 °c. determine (a) the quality of water at the initial state (b) the work associated with this process, (c) the heat associated with this process.
Answers: 2

Engineering, 06.07.2019 04:10
An inventor claims to have developed a reversed heat engine (ie. a heat pump) which is able to deliver 10 kj heat into the room at 20°c from outside ambient temperature of -10°c by consuming only 2 kj electricity in winter. this claim is a)-impossible b)-possible only if the heat pump is ideal c)-practically possible d)-unable to be assessed as it depends on the working fluid used in the heat pump.
Answers: 2

Engineering, 06.07.2019 04:10
Agas mixture with a compostion of 55 methane, 30% propane, and 15 butane by volume) is compressed from 1 bar and 25°c to 9bar in a compressor with an isentropic efficiency of 85%. the environment has a temperature of to25 c. determine (a) the exit temperature of the mixture (b) the specific work required to run the compressor (c) the specific entropy generation of the process (d) the specific exergy destruction of the process (e) the second law efficiency of the compressor
Answers: 3
You know the right answer?
Water vapor at 100 psi, 500 F and a velocity of 100 ft./sec enters a nozzle operating at steady sate...
Questions

English, 20.10.2020 04:01

History, 20.10.2020 04:01



Computers and Technology, 20.10.2020 04:01


Mathematics, 20.10.2020 04:01

SAT, 20.10.2020 04:01

History, 20.10.2020 04:01



English, 20.10.2020 04:01





Mathematics, 20.10.2020 04:01

Social Studies, 20.10.2020 04:01

Business, 20.10.2020 04:01

Chemistry, 20.10.2020 04:01