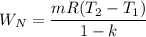Engineering, 18.06.2020 22:57 bigchriss
water at 2.0 MPa, 500 °C is brought to saturated vapor in a piston/cylinder with a reversible adiabatic process. find the final temperature and work done during this process.

Answers: 2


Another question on Engineering

Engineering, 03.07.2019 19:30
When using the ohmmeter function of a digital multimeter, the leads are placed in what position relative to the component being tested? a. parallel b. control c. series d. line
Answers: 3

Engineering, 04.07.2019 18:10
The higher the astm grain-size number, the coarser the grain is. a)-true b)-false
Answers: 3

Engineering, 04.07.2019 18:20
For each of the following process: a) sketch the p-v diagram, b)sketch t-s diagram, c) sketch t-v diagram, d) sketch the boundary work on one of the diagrams (a, b or c) and e) sketch the reversible heat transfer on one of the diagrams (a, b or c): 1- isobaric process from compressed liquid to superheated vapor 2- isothermal process from compressed liquid to superheated vapor 3- isentropic process from compressed liquid to superheated vapor
Answers: 3

Engineering, 04.07.2019 19:10
Air inially occupying a volume of 1 m2 at 100 kpa, 27 c undergoes three internally reversible processes in series. process 1-2 compression to 500 kpa during which pv constant process 2-3 adiabatic expanslon to 100 kpa process 3-1: constant-pressure expansion to 100 kpa (a) calculate the change of entropy for each of the three processes. (b) calculate the heat and work involved in each process. (c) is this cycle a power cycle or refrigeration cycle?
Answers: 3
You know the right answer?
water at 2.0 MPa, 500 °C is brought to saturated vapor in a piston/cylinder with a reversible adiaba...
Questions

Social Studies, 08.12.2021 23:00



English, 08.12.2021 23:00






Mathematics, 08.12.2021 23:00


Mathematics, 08.12.2021 23:00


English, 08.12.2021 23:00

Mathematics, 08.12.2021 23:00



Mathematics, 08.12.2021 23:00

Computers and Technology, 08.12.2021 23:00

Mathematics, 08.12.2021 23:00

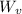 = m·
= m·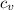 ·(T₂ - T₁)
·(T₂ - T₁)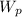 = m·
= m·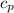 ·(T₂ - T₁)
·(T₂ - T₁)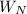 , can be expressed as follows;
, can be expressed as follows;