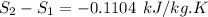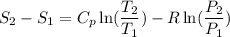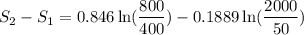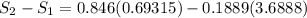Engineering, 14.07.2020 14:01 sandram74691
Carbon dioxide initially at 50 kPa, 400 K, undergoes a process in a closed system until its pressure and temperature are 2 MPa and 800 K, respectively. Assuming an ideal gas behaviour, find the entropy change of the carbon dioxide by assuming that the specific heats are constant. For the gas, take Cp = 0.846 kJ/kg. K and R = 0.1889 kJ/kg. K

Answers: 3


Another question on Engineering

Engineering, 03.07.2019 15:10
If you were designing a bumper for a car, would you prefer it to exhibit elastic or plastic deformation? why? consider the functions of a bumper in both a minor "fender-bender" and a major collision.
Answers: 1

Engineering, 04.07.2019 18:10
Abrake has a normal braking torque of 2.8 kip in and heat-dissipating cast-iron surfaces whose mass is 40 lbm. suppose a load is brought to rest in 8.0 s from an initial angular speed of 1600 rev/min using the normal braking torque; estimate the temperature rise of the heat dissipating surfaces.
Answers: 3

Engineering, 04.07.2019 18:10
Water at 70°f and streams enter the mixing chamber at the same mass flow rate, determine the temperature and the quality of the exiting stream. 0 psia is heated in a chamber by mixing it with saturated water vapor at 20 psia. if both streams enters the mixing chamber at the same mass flow rate, determine the temperature and the quality of the existing system.
Answers: 2

Engineering, 04.07.2019 18:10
Carbon dioxide gas expands isotherm a turbine from 1 mpa, 500 k at 200 kpa. assuming the ideal gas model and neglecting the kinetic and potential energies, determine the change in entropy, heat transfer and work for each kilogram of co2.
Answers: 2
You know the right answer?
Carbon dioxide initially at 50 kPa, 400 K, undergoes a process in a closed system until its pressure...
Questions

History, 28.04.2021 17:40


Mathematics, 28.04.2021 17:40

Mathematics, 28.04.2021 17:40


Mathematics, 28.04.2021 17:40

Mathematics, 28.04.2021 17:40







History, 28.04.2021 17:40





Geography, 28.04.2021 17:40

English, 28.04.2021 17:40