Engineering, 13.07.2020 21:01 NANA2007
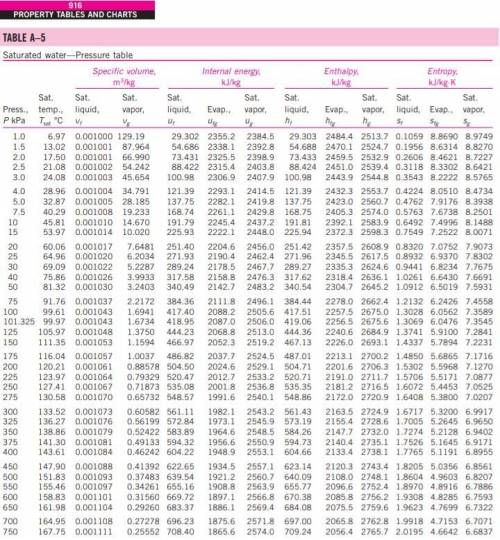
An isolated piston-cylinder device contains 5 L of saturated liquid water at a constant pressure of 150 kPa. An electric resistance heater inside the cylinder is now turned on, and 2200 kJ of energy is transferred to the steam. Determine the entropy change of the water during this process, in kJ/K.

Answers: 3


Another question on Engineering

Engineering, 03.07.2019 15:10
Two flowing streams of argon gas are adiabatically mixed to form a single flow/stream. one stream is 1.5 kg/s at 400 kpa and 200 c while the second stream is 2kg/s at 500 kpa and 100 ? . it is stated that the exit state of the mixed single flow of argon gas is 150 c and 300 kpa. assuming there is no work output or input during the mixing process, does this process violate either the first or the second law or both? explain and state all your assumptions.
Answers: 1

Engineering, 04.07.2019 18:10
Calculate the bore of a cylinder that has a stroke of 18 inches and an extension time of 6 seconds at a flow rate of 4 gal/min.
Answers: 3

Engineering, 04.07.2019 18:20
Aheavily insulated piston-cylinder device contains 0.02 m3 of steam at 300 kpa and 200 °c. 1.2 mpa. d this process. team is now compressed in a reversible manner to a pressure of etermine the entropy change and the work done on the steam during this process
Answers: 1

Engineering, 04.07.2019 19:10
Plan an experiment to measure the surface tension of a liquid similar to water. if necessary, review the ncfmf video surface tension for ideas. which method would be most suitable for use in an undergraduate laboratory? what experimental precision could be expected?
Answers: 2
You know the right answer?
An isolated piston-cylinder device contains 5 L of saturated liquid water at a constant pressure of...
Questions

Biology, 05.04.2021 16:10


Biology, 05.04.2021 16:10

Mathematics, 05.04.2021 16:10

Social Studies, 05.04.2021 16:10

World Languages, 05.04.2021 16:10

Spanish, 05.04.2021 16:10

History, 05.04.2021 16:10

Advanced Placement (AP), 05.04.2021 16:10

Physics, 05.04.2021 16:10

English, 05.04.2021 16:10



Mathematics, 05.04.2021 16:10

Mathematics, 05.04.2021 16:10

English, 05.04.2021 16:10

Chemistry, 05.04.2021 16:10