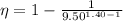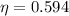Engineering, 30.08.2020 14:01 jacobdesalvo8155
Calculate the theoretical efficiency for an Otto cycle engine with γ=1.40 and compression ration r=9.50.

Answers: 1


Another question on Engineering

Engineering, 03.07.2019 14:10
Amass of m 1.5 kg of steam is contained in a closed rigid container. initially the pressure and temperature of the steam are: p 1.5 mpa and t 240°c (superheated state), respectively. then the temperature drops to t2= 100°c as the result of heat transfer to the surroundings. determine: a) quality of the steam at the end of the process, b) heat transfer with the surroundings. for: p1.5 mpa and t 240°c: enthalpy of superheated vapour is 2900 kj/kg, specific volume of superheated vapour is 0. 1483 m/kg, while for t 100°c: enthalpy of saturated liquid water is 419kj/kg, specific volume of saturated liquid water is 0.001043m/kg, enthalpy of saturated vapour is 2676 kj/kg, specific volume of saturated vapour is 1.672 m/kg and pressure is 0.1 mpa.
Answers: 3

Engineering, 04.07.2019 18:10
Steel is coated with a thin layer of ceramic to protect against corrosion. what do you expect to happen to the coating when the temperature of the steel is increased significantly? explain.
Answers: 1

Engineering, 04.07.2019 18:10
An air conditioning system consist of a 5 cm diameter pipe, operating at a pressure of 200 kpa. the air initially enters the pipe at 15°c with a velocity of 20 m/s and relative humidity of 80%. if the heat supply throughout the process is 960 w, determine the relative humidity and the temperature at the outlet
Answers: 3

Engineering, 04.07.2019 18:10
The filament of an incandescent lamp has a temperature of 2000k. calculate the fraction of radiation emitted in the visible light band if the filament is approximated as blackbody
Answers: 2
You know the right answer?
Calculate the theoretical efficiency for an Otto cycle engine with γ=1.40 and compression ration r=9...
Questions

Biology, 19.11.2020 22:00







Mathematics, 19.11.2020 22:00

Medicine, 19.11.2020 22:00




Mathematics, 19.11.2020 22:00




History, 19.11.2020 22:00

Chemistry, 19.11.2020 22:00

Mathematics, 19.11.2020 22:00

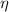 ), dimensionless, is obtained by this formula after supossing that fluid is an ideal gas:
), dimensionless, is obtained by this formula after supossing that fluid is an ideal gas: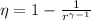
 - Compression ratio, dimensionless.
- Compression ratio, dimensionless.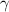 - Heat capacity ratio, dimensionless.
- Heat capacity ratio, dimensionless.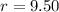 and
and 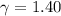 , the theoretical efficiency of the Otto engine is:
, the theoretical efficiency of the Otto engine is: