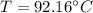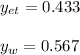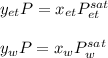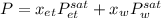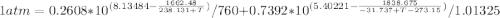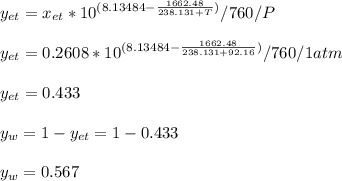Engineering, 02.09.2020 01:01 jordanrose98
Using Raoult’s law, estimate the boiling temperature and mole fractions in the vapor phase that is in equilibrium with a liquid having 0.2608 mol fraction of ethanol, in a mixture ethanol/water at P = 1 atm. If possible, solve non-numerically.

Answers: 3


Another question on Engineering

Engineering, 03.07.2019 14:10
When at a point two solid phase changes to one solid phase on cooling then it is known as a) eutectoid point b) eutectic point c) peritectic point d) peritectoid point
Answers: 3

Engineering, 04.07.2019 18:10
The mass flow rate of the fluid remains constant in all steady flow process. a)- true b)- false
Answers: 1

Engineering, 04.07.2019 18:10
For the closed feedwater heater below, feedwater enters state 3 at a pressure of 2000 psia and temperature of 420 °f at a rate of ix10 ibhr. the feedwat extracted steam enters state 1 at a pressure of 1000 psia and enthalpy of 1500 btu/lbm. the extracted er leaves at an enthalpy of 528.7 btu/lbm steam leaves as a saturated liquid. (16) a) determine the mass flow rate of the extraction steam used to heat the feedwater (10) b) determine the terminal temperature difference of the closed feedwater heater
Answers: 3

Engineering, 04.07.2019 18:10
Water at 55c flows across a flat plate whose surface temperature is held constant at 95c. if the temperature gradient at the plate's surface for a given value of x is 18 c/mm, find a) local heat transfer coefficient. b) heat flux
Answers: 3
You know the right answer?
Using Raoult’s law, estimate the boiling temperature and mole fractions in the vapor phase that is i...
Questions

Computers and Technology, 11.10.2020 23:01
















Mathematics, 11.10.2020 23:01