Engineering, 09.12.2020 18:40 cache77
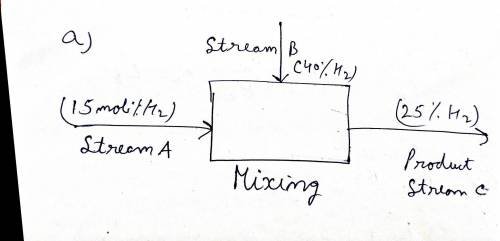
A gas stream (A) of 15.0 mol% H2, and the balance N2, is to be mixed with another gas stream
(B) containing 40.0 mol% H2, and the balance N2, to produce 100 kg/h of a 25 mol% H2, and the
balance N2 gas stream (C).
(a) Draw and fully label a flowchart of the mixing process.
(b) Calculate the average molecular weight of the product stream (C).
(c) Calculate the molar flow rates of the product stream (C) in kmol/h.
(d) Calculate the required flow rates of the feed mixtures A and B in kmol/h.

Answers: 3


Another question on Engineering

Engineering, 04.07.2019 18:10
Abrake has a normal braking torque of 2.8 kip in and heat-dissipating cast-iron surfaces whose mass is 40 lbm. suppose a load is brought to rest in 8.0 s from an initial angular speed of 1600 rev/min using the normal braking torque; estimate the temperature rise of the heat dissipating surfaces.
Answers: 3

Engineering, 04.07.2019 18:10
During a steady flow process, the change of energy with respect to time is zero. a)- true b)- false
Answers: 2

Engineering, 04.07.2019 18:10
Items are similar to the free issue items, but their access is limited. (clo5) a)-bin stock items free issue b)-bin stock controlled issue c)-critical or insurance spares d)-rebuildable spares e)-consumables
Answers: 1

Engineering, 04.07.2019 19:10
The proportional limit is always greater than the yield strength for a material. a)-trune b)- false
Answers: 3
You know the right answer?
A gas stream (A) of 15.0 mol% H2, and the balance N2, is to be mixed with another gas stream
(B) co...
Questions








Mathematics, 04.10.2020 14:01



English, 04.10.2020 14:01


English, 04.10.2020 14:01

Health, 04.10.2020 14:01

English, 04.10.2020 14:01




Arts, 04.10.2020 14:01