
Engineering, 17.12.2020 17:40 Carriepruitt
An ideal gas, consisting of n moles, undergoes an irreversible process in which the temperature has the same value at the beginning and end. If the volume changes from Vi to Vf , the change in entropy is given by:

Answers: 3


Another question on Engineering

Engineering, 04.07.2019 18:10
Acompressor receives the shaft work to decrease the pressure of the fluid. a)- true b)- false
Answers: 3

Engineering, 04.07.2019 18:10
An air conditioning system consist of a 5 cm diameter pipe, operating at a pressure of 200 kpa. the air initially enters the pipe at 15°c with a velocity of 20 m/s and relative humidity of 80%. if the heat supply throughout the process is 960 w, determine the relative humidity and the temperature at the outlet
Answers: 3

Engineering, 04.07.2019 18:10
Refrigerant 134a enters an insulated compressor operating at steady state as saturated vapor at -26°c with a volumetric flow rate of 0.18 m3/s. refrigerant exits at 9 bar, 70°c. changes in kinetic and potential energy from inlet to exit can be ignored. determine the volumetric flow rate at the exit, in m3/s, and the compressor power, in kw.
Answers: 1

Engineering, 04.07.2019 18:10
Thermal stresses are developed in a metal when its a) initial temperature is changed b) final temperature is changed c) density is changed d) thermal deformation is prevented e) expansion is prevented f) contraction is prevented
Answers: 2
You know the right answer?
An ideal gas, consisting of n moles, undergoes an irreversible process in which the temperature has...
Questions

Health, 12.12.2020 16:40

English, 12.12.2020 16:40

English, 12.12.2020 16:40









Mathematics, 12.12.2020 16:40

Health, 12.12.2020 16:40



Mathematics, 12.12.2020 16:40


Computers and Technology, 12.12.2020 16:40


Arts, 12.12.2020 16:40

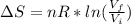
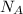 k, k is Boltzmann's constant in J K⁻¹ and Avogadro's constant
k, k is Boltzmann's constant in J K⁻¹ and Avogadro's constant 

