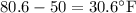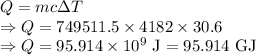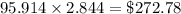Engineering, 18.12.2020 17:00 verawall39411
Thermodynamics fill in the blanks The swimming pool at the local YMCA holds roughly 749511.5 L (749511.5 kg) of water and is kept at a temperature of 80.6 °F year round using a natural gas heater. If you were to completely drain the pool and refill the pool with 50°F water, (blank) GJ (giga-Joules) of energy are required to to heat the water back to 80.6 °F. Note: The specific heat capacity of water is 4182 J/kg ⋅°C. The cost of natural gas per GJ is $2.844. It costs $ (blank) to heat the pool (to the nearest dollar).

Answers: 1


Another question on Engineering

Engineering, 04.07.2019 12:10
On a average work day more than work place firs are reorted
Answers: 1

Engineering, 04.07.2019 19:10
How to increase the thermal officiency of an ideal simple rankino cycle? among these methods, which one is the best and why?
Answers: 2

Engineering, 04.07.2019 19:10
Estimate the change in specific internal energy au and specific enthalpy h from inlet to outlet for ethylene glycol (a liquid) flowing through each of the following devices: (a) a heat exchanger where the glycol temperature increases from 20 °c to 80 °c; (b) a pump operating at about 25 °c and increasing the glycol pressure from 100 kpa to 8 mpa.
Answers: 2

Engineering, 04.07.2019 19:10
Agas is compressed from vi 0.3 m, p 1 bar to of v2 0.1 m3, p2--3 bar. pressure and volume are related linearly during the process. for the gas, find the work, in kj.
Answers: 2
You know the right answer?
Thermodynamics fill in the blanks The swimming pool at the local YMCA holds roughly 749511.5 L (7495...
Questions


Mathematics, 13.01.2021 06:40


Mathematics, 13.01.2021 06:40

Mathematics, 13.01.2021 06:40

Physics, 13.01.2021 06:40



Mathematics, 13.01.2021 06:40

Mathematics, 13.01.2021 06:40


Mathematics, 13.01.2021 06:40

Biology, 13.01.2021 06:40







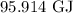
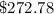
 = Change in temperature =
= Change in temperature =