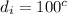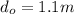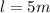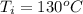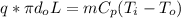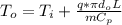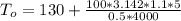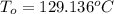Engineering, 17.06.2021 21:00 lindsaypre0644
Consider the same piping system. this time, the same pipe is buried underground. assuming that there is a constant heat flux of 100w/m^2 from the outer surfance of the pipe to the soil determine the exit temperature of the water.
a. 129.1
b. 111.1
c. 82.1
d. 68.1

Answers: 3


Another question on Engineering

Engineering, 04.07.2019 18:10
Apump is used to circulate hot water in a home heating system. water enters the well-insulated pump operating at steady state at a rate of 0.42 gal/min. the inlet pressure and temperature are 14.7 lbf/in.2, and 180°f, respectively; at the exit the pressure is 60 lbf/in.2 the pump requires 1/15 hp of power input. water can be modeled as an incompressible substance with constant density of 60.58 lb/ft3 and constant specific heat of 1 btu/lb or. neglecting kinetic and potential energy effects, determine the temperature change, in °r, as the water flows through the pump.
Answers: 1

Engineering, 04.07.2019 18:20
Most leaks in reciprocating air compressors can be detected and minimized by: (clo4) a)-detecting leakage areas using ultrasonic acoustic detector. b)-tightening joints and connections c)-replacing faulty equipment d)-all of the given options
Answers: 2

Engineering, 04.07.2019 18:20
Ahe-xe mixture containing a 0.75 mole fraction of helium is used for cooling electronics in an avionics application. at a temperature of 300 k and atmospheric pressure, calculate the mass fraction of helium and the mass density, molar concentration and molecular weight of the mixture. if the cooling capacity is 10 l, what is the mass of the coolant?
Answers: 3

Engineering, 04.07.2019 19:10
What is the major difference between thermoplastics and thermosetting plastics from the polymerization structure point of view?
Answers: 2
You know the right answer?
Consider the same piping system. this time, the same pipe is buried underground. assuming that there...
Questions

English, 24.10.2019 00:50


Mathematics, 24.10.2019 00:50


Mathematics, 24.10.2019 00:50














Biology, 24.10.2019 00:50