
Mathematics, 23.06.2019 23:00 juliannasl
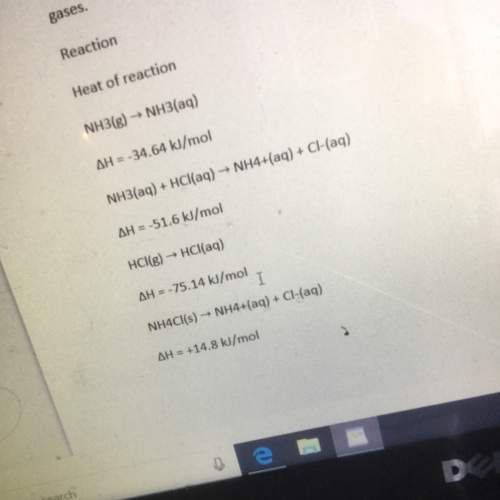
The heat of reaction for the process described in (a) can be determined by applying hess’s law. the heats of reaction shown in the table below can be obtained experimentally or looked up in tables of enthalpy data which two of these heats of reaction would be the easiest and safest to measure in the laboratory and which two are better obtained through reference sources why ? hint: consider whether a reaction takes place in aqueous solution or instead involves noxious gases. table is below

Answers: 3


Another question on Mathematics

Mathematics, 21.06.2019 16:30
Jackie purchased 3 bottles of water and 2 cups of coffee for a family for $7.35. brian bought 4 bottles of water and 1 cup of coffee for his family for $7.15. how much does each bottle of water cost? how much does each cup of coffee cost? i need this done
Answers: 3

Mathematics, 21.06.2019 20:30
Drag the tiles to the correct boxes to complete the pairs. not all tiles will be used. match each division expression with the correct quotient.
Answers: 2

Mathematics, 21.06.2019 22:00
Fatima plans to spend at least $15 and at most $20 dollars on sketch pads and pencils. if she buys 2 sketchpads, how many pemcils can she buy while staying in her price range? fatima can buy between and pencils. ? (type whole numbers. use ascending? order.)
Answers: 1

You know the right answer?
The heat of reaction for the process described in (a) can be determined by applying hess’s law. the...
Questions

History, 12.02.2021 14:40

English, 12.02.2021 14:40

Mathematics, 12.02.2021 14:40

Mathematics, 12.02.2021 14:40


Mathematics, 12.02.2021 14:40

Geography, 12.02.2021 14:40


Computers and Technology, 12.02.2021 14:40

Mathematics, 12.02.2021 14:40


English, 12.02.2021 14:50

Mathematics, 12.02.2021 14:50




Mathematics, 12.02.2021 14:50


Mathematics, 12.02.2021 14:50

History, 12.02.2021 14:50



