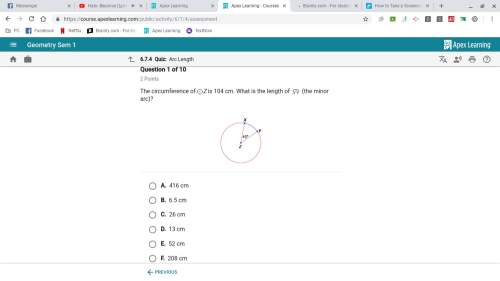
Mathematics, 30.06.2019 04:10 keenonwhite6801
The compound cd undergoes dissociation in aqueous solution to produce c and d ions. at equilibrium (25 c), the concentrations of the both ions is 1.22x 10 m. given the standard enthalpy for this dissociation is 22.2 kj/mol, calculate the equilibrium constants for this dissociation process at 25 "c and 50 °c.

Answers: 1


Another question on Mathematics

Mathematics, 21.06.2019 18:30
If you are trying to move an attached term across the equal sign (=), what operation would you use to move it?
Answers: 2

Mathematics, 22.06.2019 00:30
Given abc find the values of x and y. in your final answer, include all of your calculations.
Answers: 1

Mathematics, 22.06.2019 06:40
Find the measure of angle x. a. 11° b. 12° c. 17° d. 61°
Answers: 1

You know the right answer?
The compound cd undergoes dissociation in aqueous solution to produce c and d ions. at equilibrium (...
Questions



Mathematics, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01


English, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01


Mathematics, 13.10.2020 02:01


World Languages, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01


Biology, 13.10.2020 02:01

Social Studies, 13.10.2020 02:01


Mathematics, 13.10.2020 02:01

History, 13.10.2020 02:01


Mathematics, 13.10.2020 02:01