Mathematics, 01.11.2019 08:31 oliviavaughan01
The ideal gas law states that the volume, v, of a gas in liters varies directly with the amount of the gas in moles, n, and the absolute temperature in kelvin, t, and varies inversely with the pressure, p, of the gas. two moles of a gas has a volume of 35.424 l at 270 kelvin when p = 1.25 atm. what is the volume of 3 moles of a gas at 280 kelvin when p = 1.5 atm?
a.11.48 l
b.27.33 l
c.45.92 l
d.103.32 l

Answers: 2


Another question on Mathematics

Mathematics, 21.06.2019 18:20
What are the solution(s) to the quadratic equation x2 – 25 = 0? o x = 5 and x = -5ox=25 and x = -25o x = 125 and x = -125o no real solution
Answers: 2


Mathematics, 21.06.2019 22:30
An ant travels at a constant rate of 30cm every 2 minutes.at what speed does the ant travel per minute
Answers: 2

Mathematics, 21.06.2019 23:00
Susie wants to take her friend out for lunch.she wants to spend $9.75 each person. how much will she spend in all.
Answers: 2
You know the right answer?
The ideal gas law states that the volume, v, of a gas in liters varies directly with the amount of t...
Questions


English, 16.11.2020 20:40


Mathematics, 16.11.2020 20:40


History, 16.11.2020 20:40

Physics, 16.11.2020 20:40

English, 16.11.2020 20:40

History, 16.11.2020 20:40

Arts, 16.11.2020 20:40



Social Studies, 16.11.2020 20:40





Health, 16.11.2020 20:40


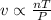
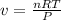 Equation 1
Equation 1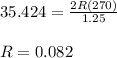
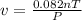 Equation 2
Equation 2